26. Statement 1: Besides its value for jewelry, currency, and electronics, gold is also important in the health professions. Statement 2: Salts of Aut are used to treat certain types of rheumatoid arthritis. 27. Statement 1: Cellular respiration allows orn=ganisms to liberate the energy stored in the chemical bonds of glucose. Statement 2: C6H12O6 + 6 026 CO2 + 6 H₂O + 36 ATP; In this reaction, glucose is reduced and oxygen is oxidized. 28. Statement 1: The reaction of a metal with either an acid or a metal salt is called a displacement reaction because the ion in the solution is replaced through the reduction of an element. Statement 2: When metals undergo displacement reactions with acids, salts and hydrogen gas is produced.
26. Statement 1: Besides its value for jewelry, currency, and electronics, gold is also important in the health professions. Statement 2: Salts of Aut are used to treat certain types of rheumatoid arthritis. 27. Statement 1: Cellular respiration allows orn=ganisms to liberate the energy stored in the chemical bonds of glucose. Statement 2: C6H12O6 + 6 026 CO2 + 6 H₂O + 36 ATP; In this reaction, glucose is reduced and oxygen is oxidized. 28. Statement 1: The reaction of a metal with either an acid or a metal salt is called a displacement reaction because the ion in the solution is replaced through the reduction of an element. Statement 2: When metals undergo displacement reactions with acids, salts and hydrogen gas is produced.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section20.2: The Aqua Sphere (water)
Problem 2RC
Related questions
Question
Please answer all thank youu
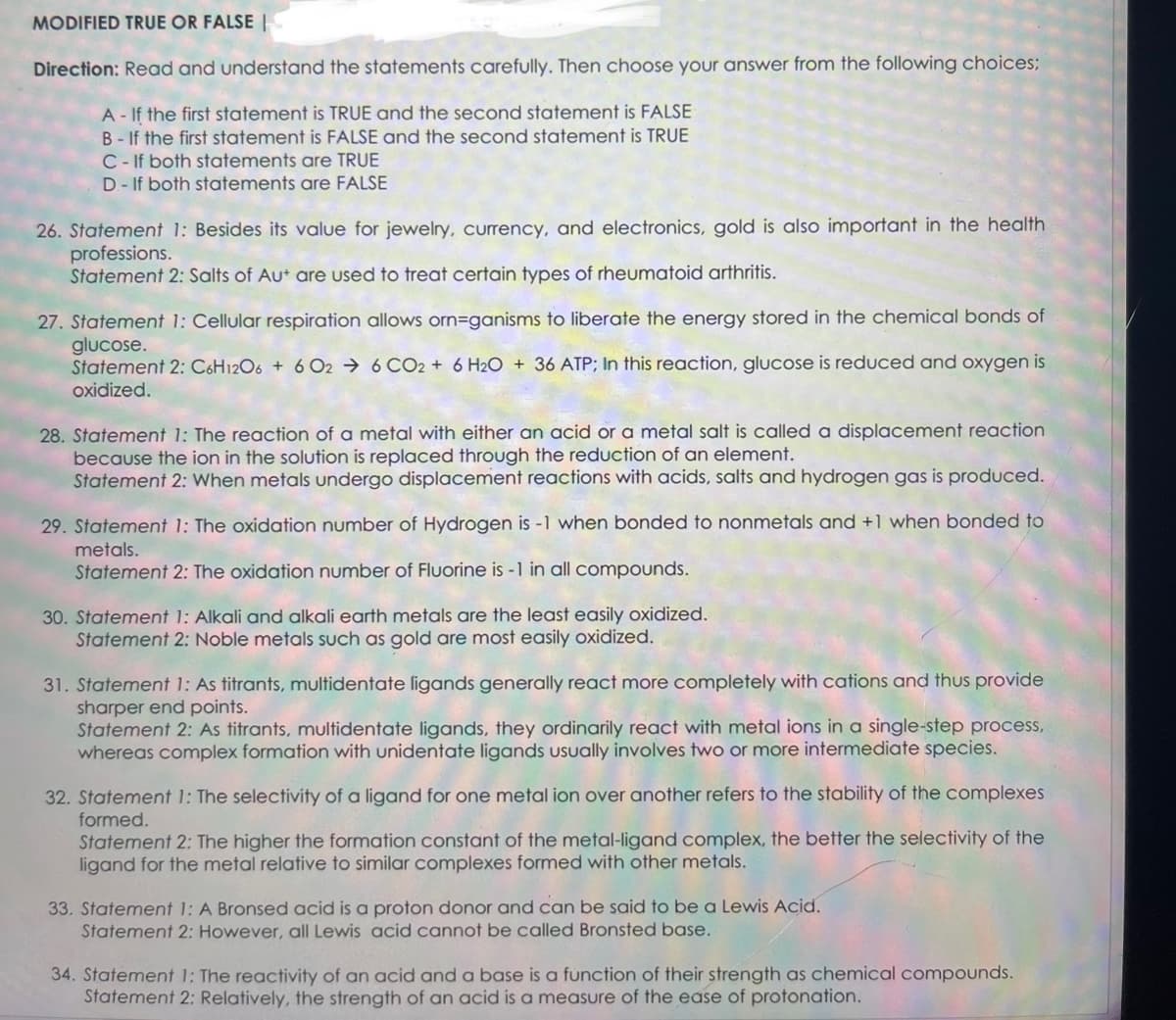
Transcribed Image Text:MODIFIED TRUE OR FALSE
Direction: Read and understand the statements carefully. Then choose your answer from the following choices;
A - If the first statement is TRUE and the second statement is FALSE
B-If the first statement is FALSE and the second statement is TRUE
C-If both statements are TRUE
D- If both statements are FALSE
26. Statement 1: Besides its value for jewelry, currency, and electronics, gold is also important in the health
professions.
Statement 2: Salts of Aut are used to treat certain types of rheumatoid arthritis.
27. Statement 1: Cellular respiration allows orn=ganisms to liberate the energy stored in the chemical bonds of
glucose.
Statement 2: C6H12O6 + 6 026 CO2 + 6H2O + 36 ATP; In this reaction, glucose is reduced and oxygen is
oxidized.
28. Statement 1: The reaction of a metal with either an acid or a metal salt is called a displacement reaction
because the ion in the solution is replaced through the reduction of an element.
Statement 2: When metals undergo displacement reactions with acids, salts and hydrogen gas is produced.
29. Statement 1: The oxidation number of Hydrogen is -1 when bonded to nonmetals and +1 when bonded to
metals.
Statement 2: The oxidation number of Fluorine is -1 in all compounds.
30. Statement 1: Alkali and alkali earth metals are the least easily oxidized.
Statement 2: Noble metals such as gold are most easily oxidized.
31. Statement 1: As titrants, multidentate ligands generally react more completely with cations and thus provide
sharper end points.
Statement 2: As titrants, multidentate ligands, they ordinarily react with metal ions in a single-step process,
whereas complex formation with unidentate ligands usually involves two or more intermediate species.
32. Statement 1: The selectivity of a ligand for one metal ion over another refers to the stability of the complexes
formed.
Statement 2: The higher the formation constant of the metal-ligand complex, the better the selectivity of the
ligand for the metal relative to similar complexes formed with other metals.
33. Statement 1: A Bronsed acid is a proton donor and can be said to be a Lewis Acid.
Statement 2: However, all Lewis acid cannot be called Bronsted base.
34. Statement 1: The reactivity of an acid and a base is a function of their strength as chemical compounds.
Statement 2: Relatively, the strength of an acid is a measure of the ease of protonation.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning