As a chemist for an agricultural products company, you have just developed a new herbicide,"Herbigon," that you think has the potential to kill weeds effectively. A sparingly soluble salt, Herbigon is dissolved in 1 M acetic acid for technical reasons having to do with its production. You have determined that the solubility product Ksp of Herbigon is 8.70x10-6. Although the formula of this new chemical is a trade secret, it can be revealed that the formula for Herbigon is X-acetate (XCH3COO, where "X" represents the top-secret cation of the salt). It is this cation that kills weeds. Since it is critical to have Herbigon dissolved (it won't kill weeds as a suspension), you are working on adjusting the pH so Herbigon will be soluble at the concentration needed to kill weeds. What pH must the solution have to yield a solution in which the concentration of X is 2.00x10-3 M? The pK, of acetic acid is 4.76. Express your answer numerically.
As a chemist for an agricultural products company, you have just developed a new herbicide,"Herbigon," that you think has the potential to kill weeds effectively. A sparingly soluble salt, Herbigon is dissolved in 1 M acetic acid for technical reasons having to do with its production. You have determined that the solubility product Ksp of Herbigon is 8.70x10-6. Although the formula of this new chemical is a trade secret, it can be revealed that the formula for Herbigon is X-acetate (XCH3COO, where "X" represents the top-secret cation of the salt). It is this cation that kills weeds. Since it is critical to have Herbigon dissolved (it won't kill weeds as a suspension), you are working on adjusting the pH so Herbigon will be soluble at the concentration needed to kill weeds. What pH must the solution have to yield a solution in which the concentration of X is 2.00x10-3 M? The pK, of acetic acid is 4.76. Express your answer numerically.
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
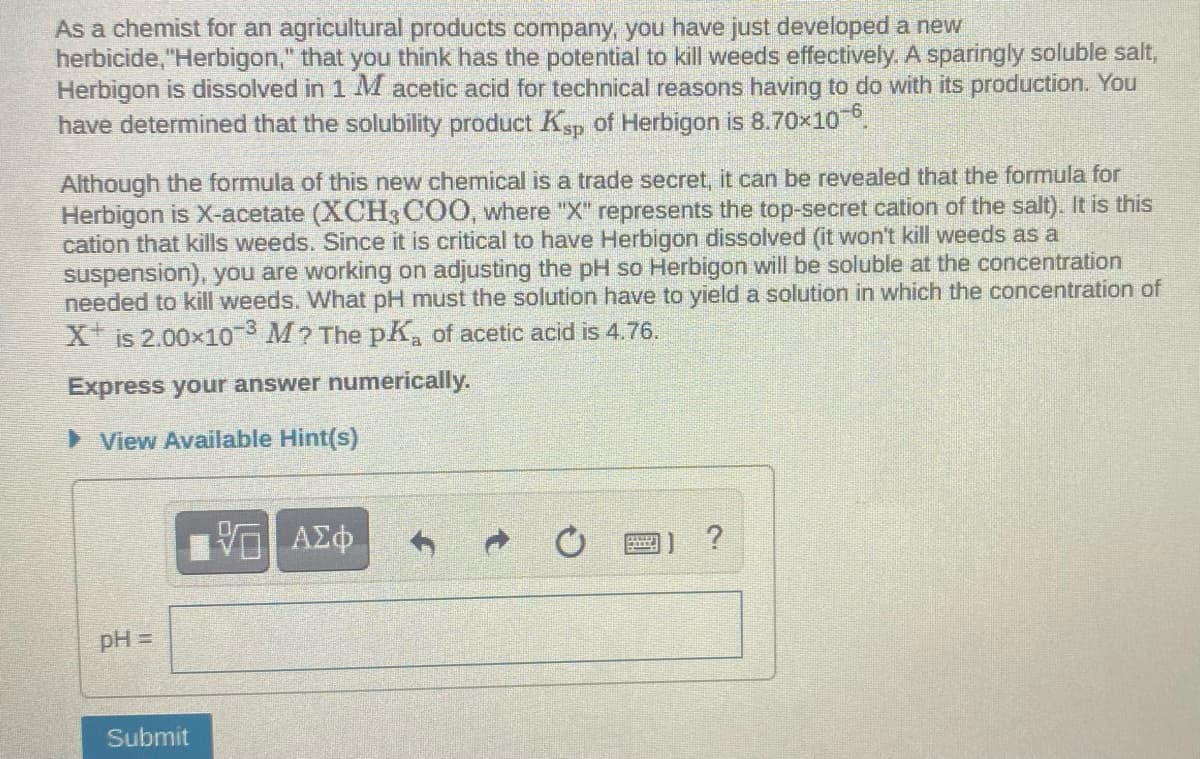
Transcribed Image Text:As a chemist for an agricultural products company, you have just developed a new
herbicide, "Herbigon," that you think has the potential to kill weeds effectively. A sparingly soluble salt,
Herbigon is dissolved in 1 M acetic acid for technical reasons having to do with its production. You
have determined that the solubility product Ksp of Herbigon is 8.70x10.
Although the formula of this new chemical is a trade secret, it can be revealed that the formula for
Herbigon is X-acetate (XCH3 COO, where "X" represents the top-secret cation of the salt). It is this
cation that kills weeds. Since it is critical to have Herbigon dissolved (it won't kill weeds as a
suspension), you are working on adjusting the pH so Herbigon will be soluble at the concentration
needed to kill weeds. What pH must the solution have to yield a solution in which the concentration of
X is 2.00x10 M? The pK, of acetic acid is 4.76.
Express your answer numerically.
> View Available Hint(s)
pH =
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning