I2 is considerably more soluble in CC14 (1) than it is in H2O(1). At a certain temperature, the concentration of I, in its saturated aqueous solution is 1.300 x 10-3 M and the equilibrium achieved when I2 distributes itself between H2O and CCl, is A 12.0-mL sample of saturated I2(aq) is shaken with 12.0 mL of CC14. After equilibrium is established, the two liquid layers are separated. How many milligrams of I2 will be in the aqueous layer? Express your answer to two significant figures and include the appropriate units. > View Available Hint(s) I2(aq) =12(CC4), K=85.5 Submit Previous Answers Completed Part B If the 12.0-mL sample of aqueous layer from Part A is extracted with a second 12.0-mL portion of CCL4, how many milligram of I2 will remain in the aqueous layer when equilibrium is reestablished?
I2 is considerably more soluble in CC14 (1) than it is in H2O(1). At a certain temperature, the concentration of I, in its saturated aqueous solution is 1.300 x 10-3 M and the equilibrium achieved when I2 distributes itself between H2O and CCl, is A 12.0-mL sample of saturated I2(aq) is shaken with 12.0 mL of CC14. After equilibrium is established, the two liquid layers are separated. How many milligrams of I2 will be in the aqueous layer? Express your answer to two significant figures and include the appropriate units. > View Available Hint(s) I2(aq) =12(CC4), K=85.5 Submit Previous Answers Completed Part B If the 12.0-mL sample of aqueous layer from Part A is extracted with a second 12.0-mL portion of CCL4, how many milligram of I2 will remain in the aqueous layer when equilibrium is reestablished?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 37P
Related questions
Question
1
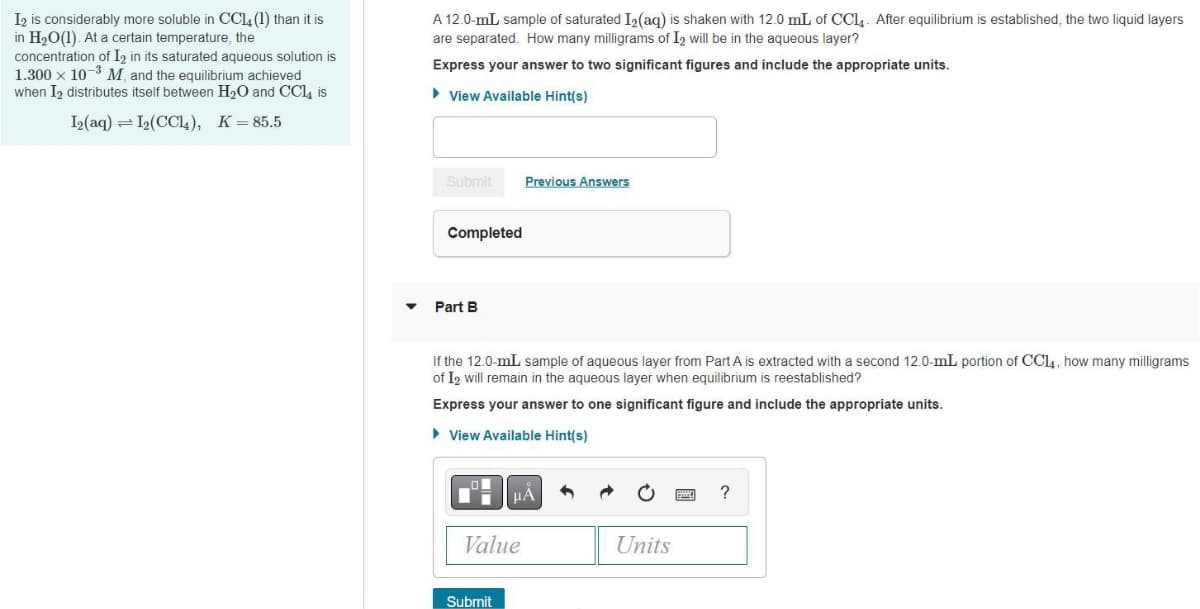
Transcribed Image Text:I2 is considerably more soluble in CC14 (1) than it is
in H20(1). At a certain temperature, the
concentration of I2 in its saturated aqueous solution is
1.300 x 10-3 M and the equilibrium achieved
when I2 distributes itself between H2O and CCl, is
A 12.0-mL sample of saturated I,(aq) is shaken with 12.0 mL of CCI4. After equilibrium is established, the two liquid layers
are separated. How many milligrams of I2 will be in the aqueous layer?
Express your answer to two significant figures and include the appropriate units.
> View Available Hint(s)
I2(aq) = I2(CCl,), K=85.5
Submit
Previous Answers
Completed
Part B
If the 12.0-mL sample of aqueous layer from Part A is extracted with a second 12.0-mL portion of CCL4, how many milligrams
of I2 will remain in the aqueous layer when equilibrium is reestablished?
Express your answer to one significant figure and include the appropriate units.
> View Available Hint(s)
Value
Units
Submit
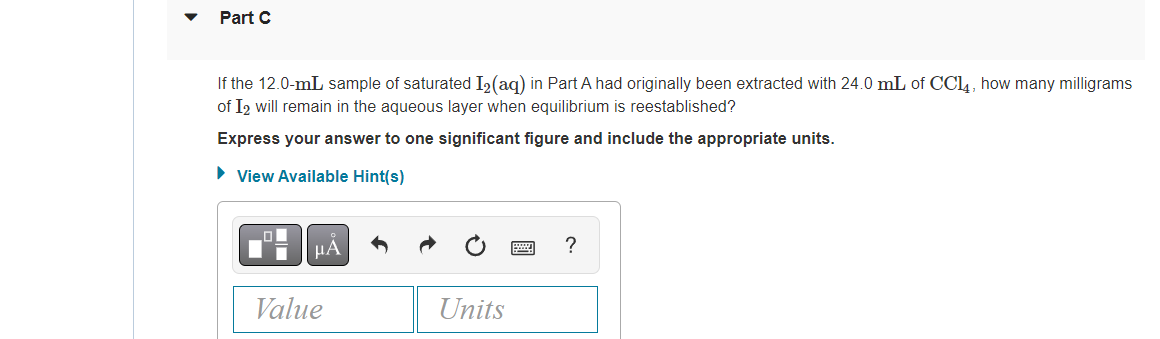
Transcribed Image Text:Part C
If the 12.0-mL sample of saturated I2(aq) in Part A had originally been extracted with 24.0 mL of CCL4, how many milligrams
of I2 will remain in the aqueous layer when equilibrium is reestablished?
Express your answer to one significant figure and include the appropriate units.
• View Available Hint(s)
HÀ
?
Value
Units
國
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning