Ascorbic acid, H2C6H6O6, is a diprotic acid usually known as vitamin C. For this acid, pKa1 is 4.10 and pKa2 is 11.79. When 125 mL of a solution of ascorbic acid was evaporated to dryness, the residue of pure ascorbic acid had a mass of 3.64 g. Your answer is correct. Calculate the molar concentration of ascorbic acid in the solution before it was evaporated. 0.164 Hint Your answer is partially correct. What was the pH of the ascorbic acid solution before it was evaporated? pH = 2.44 What was the concentration of the ascorbate ion, C6H6062, before the solution was evaporated? i M M
Ascorbic acid, H2C6H6O6, is a diprotic acid usually known as vitamin C. For this acid, pKa1 is 4.10 and pKa2 is 11.79. When 125 mL of a solution of ascorbic acid was evaporated to dryness, the residue of pure ascorbic acid had a mass of 3.64 g. Your answer is correct. Calculate the molar concentration of ascorbic acid in the solution before it was evaporated. 0.164 Hint Your answer is partially correct. What was the pH of the ascorbic acid solution before it was evaporated? pH = 2.44 What was the concentration of the ascorbate ion, C6H6062, before the solution was evaporated? i M M
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 119QRT
Related questions
Question
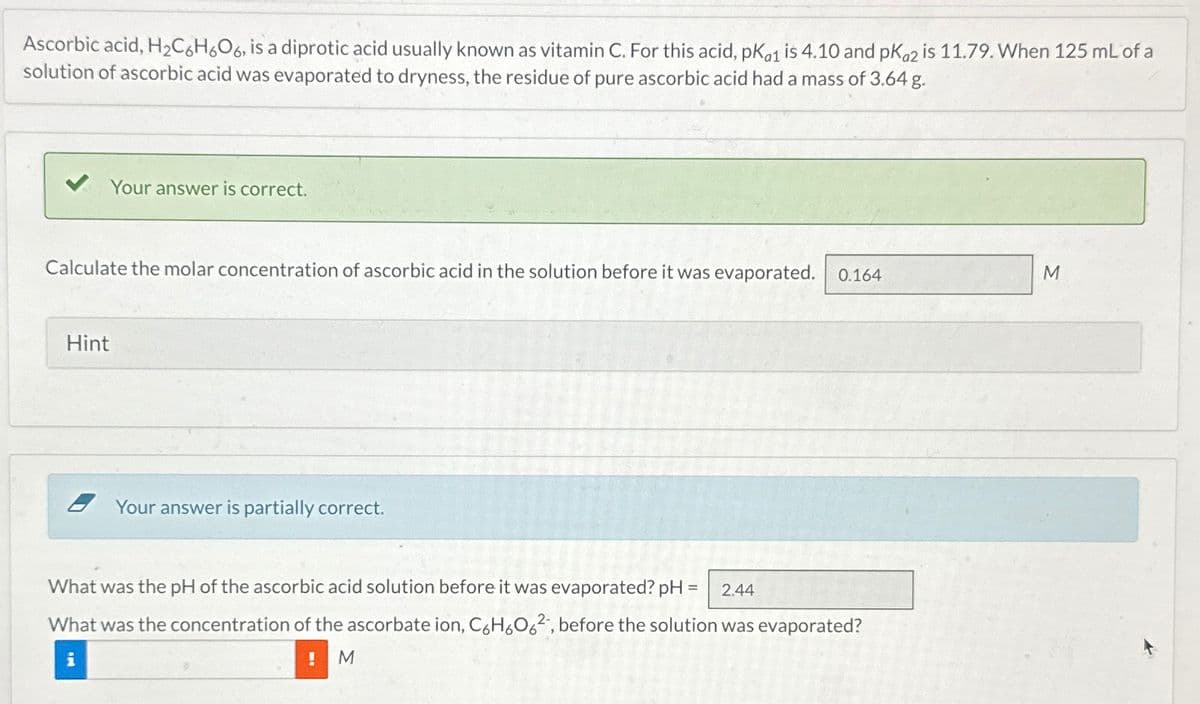
Transcribed Image Text:Ascorbic acid, H2C6H6O6, is a diprotic acid usually known as vitamin C. For this acid, pKa1 is 4.10 and pKa2 is 11.79. When 125 mL of a
solution of ascorbic acid was evaporated to dryness, the residue of pure ascorbic acid had a mass of 3.64 g.
Your answer is correct.
Calculate the molar concentration of ascorbic acid in the solution before it was evaporated. 0.164
Hint
Your answer is partially correct.
What was the pH of the ascorbic acid solution before it was evaporated? pH = 2.44
What was the concentration of the ascorbate ion, C6H6062, before the solution was evaporated?
i
M
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning