ASK YOUR TEACH A new liquid substance has been discovered that freezes at 44°C and has a latent heat of 471,000 3/kg when freezing. A scientist poured the liquid (which was at 44C) into a 0.711 kg cup (Coup=924 3/kg-K) which caused some, but not all, of the liquid to freeze. As a result, the liquid lost 67,000 3 of heat until thermal equilibrium was reached Determine how much of the liquid froze & the initial temperature of the cup. mfreezes Teup, °℃ MY NOTES
ASK YOUR TEACH A new liquid substance has been discovered that freezes at 44°C and has a latent heat of 471,000 3/kg when freezing. A scientist poured the liquid (which was at 44C) into a 0.711 kg cup (Coup=924 3/kg-K) which caused some, but not all, of the liquid to freeze. As a result, the liquid lost 67,000 3 of heat until thermal equilibrium was reached Determine how much of the liquid froze & the initial temperature of the cup. mfreezes Teup, °℃ MY NOTES
College Physics
10th Edition
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter10: Thermal Physics
Section: Chapter Questions
Problem 7WUE: One way to cool a gas is to let it expand. When a certain gas under a pressure of 5.00 106 Ha at...
Related questions
Question
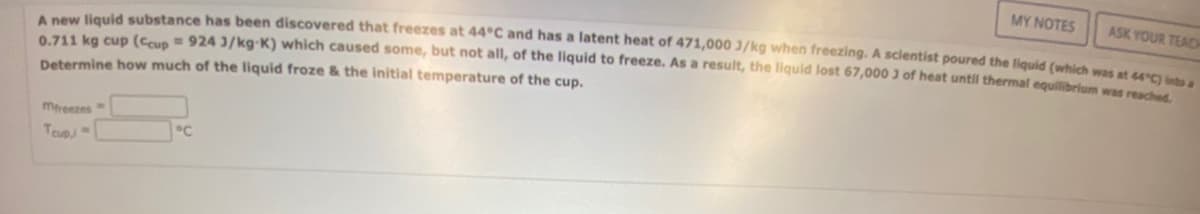
Transcribed Image Text:A new liquid substance has been discovered that freezes at 44°C and has a latent heat of 471,000 J/kg when freezing. A scientist poured the liquid (which was at 44°C) into a
0.711 kg cup (Ccup = 924 3/kg-K) which caused some, but not all, of the liquid to freeze. As a result, the liquid lost 67,000 3 of heat until thermal equilibrium was reached.
Determine how much of the liquid froze & the initial temperature of the cup.
mfreezes
Teup,
°℃
MY NOTES
ASK YOUR TEACH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning