Assess the energy balance of all the sub-systems in the block diagram below and state all the assumptions used with suitable justifications. X Solution (22% Water) Ethanol-water (22% of Water) Water Evaporator Concen. X ethanol Concen. Y (benzene) Mixing Tank Binary XY Ethanol- Benzene 1933 kg/h Oven (298.15 K) Binary XY Ethanol- benzene X (Ethanol) Distillation (340.90 K) Y (Benzene)
Assess the energy balance of all the sub-systems in the block diagram below and state all the assumptions used with suitable justifications. X Solution (22% Water) Ethanol-water (22% of Water) Water Evaporator Concen. X ethanol Concen. Y (benzene) Mixing Tank Binary XY Ethanol- Benzene 1933 kg/h Oven (298.15 K) Binary XY Ethanol- benzene X (Ethanol) Distillation (340.90 K) Y (Benzene)
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
J 5
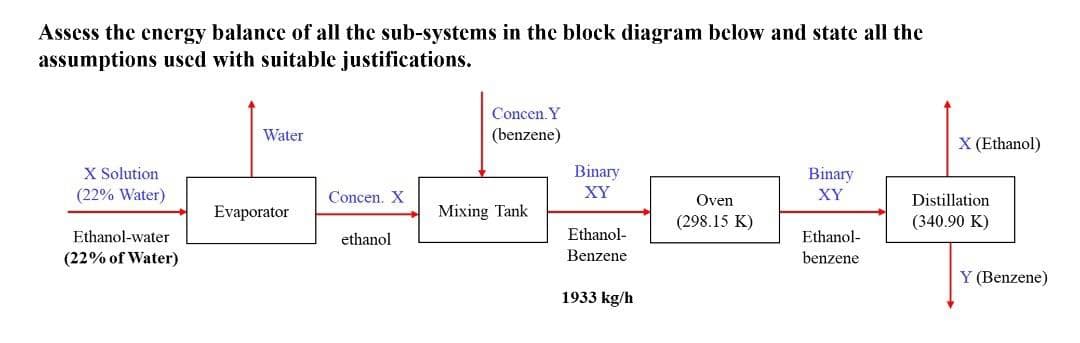
Transcribed Image Text:Assess the energy balance of all the sub-systems in the block diagram below and state all the
assumptions used with suitable justifications.
Concen. Y
Water
(benzene)
X (Ethanol)
X Solution
Binary
Binary
stogod
(22% Water)
Concen. X
XY
Oven
XY
Distillation.
Evaporator
Mixing Tank
(298.15 K)
(340.90 K)
Ethanol-water
ethanol
Ethanol-
Ethanol-
(22% of Water)
Benzene
benzene
Y (Benzene)
1933 kg/h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The