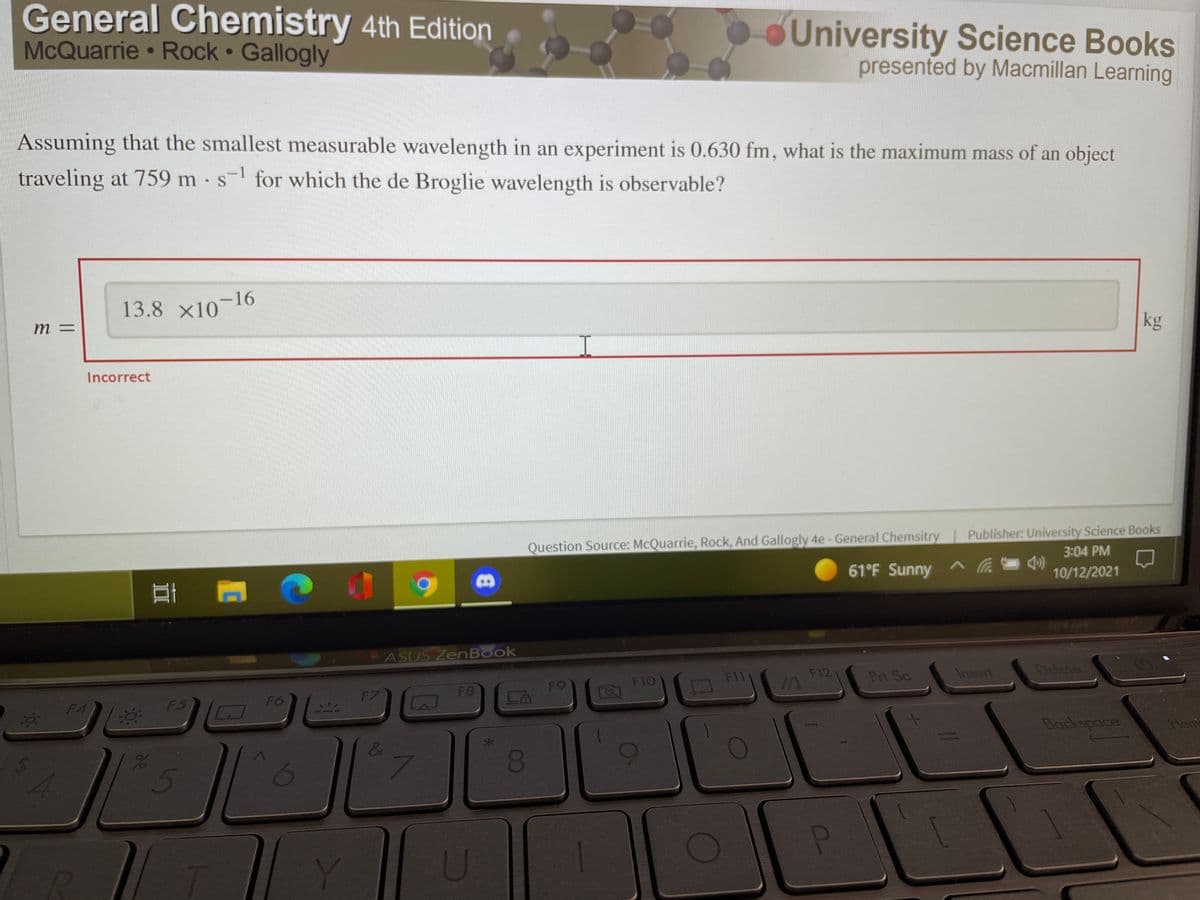
Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object traveling at 759 m s for which the de Broglie wavelength is observable? 13.8 x10-16 kg m = Incorrect
Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object traveling at 759 m s for which the de Broglie wavelength is observable? 13.8 x10-16 kg m = Incorrect
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 17P: In a FranckHertz experiment on sodium atoms, the first excitation threshold occurs at 2.103 eV....
Related questions
Question
How to do this
Transcribed Image Text:General Chemistry 4th Edition
McQuarrie • Rock • Gallogly
University Science Books
presented by Macmillan Learning
Assuming that the smallest measurable wavelength in an experiment is 0.630 fm, what is the maximum mass of an object
traveling at 759 m s- for which the de Broglie wavelength is observable?
13.8 x10-16
m =
kg
Incorrect
Question Source: McQuarrie, Rock, And Gallogly 4e-General Chemsitry Publisher: University Science Books
3:04 PM
61°F Sunny ^
10/12/2021
ASUS Zen Book
F11
F12
IA
Delota
F9
F10
Prt Sc
Insert
F6
F7
F8
F4
F5
వెండఅంంల
Hont
&
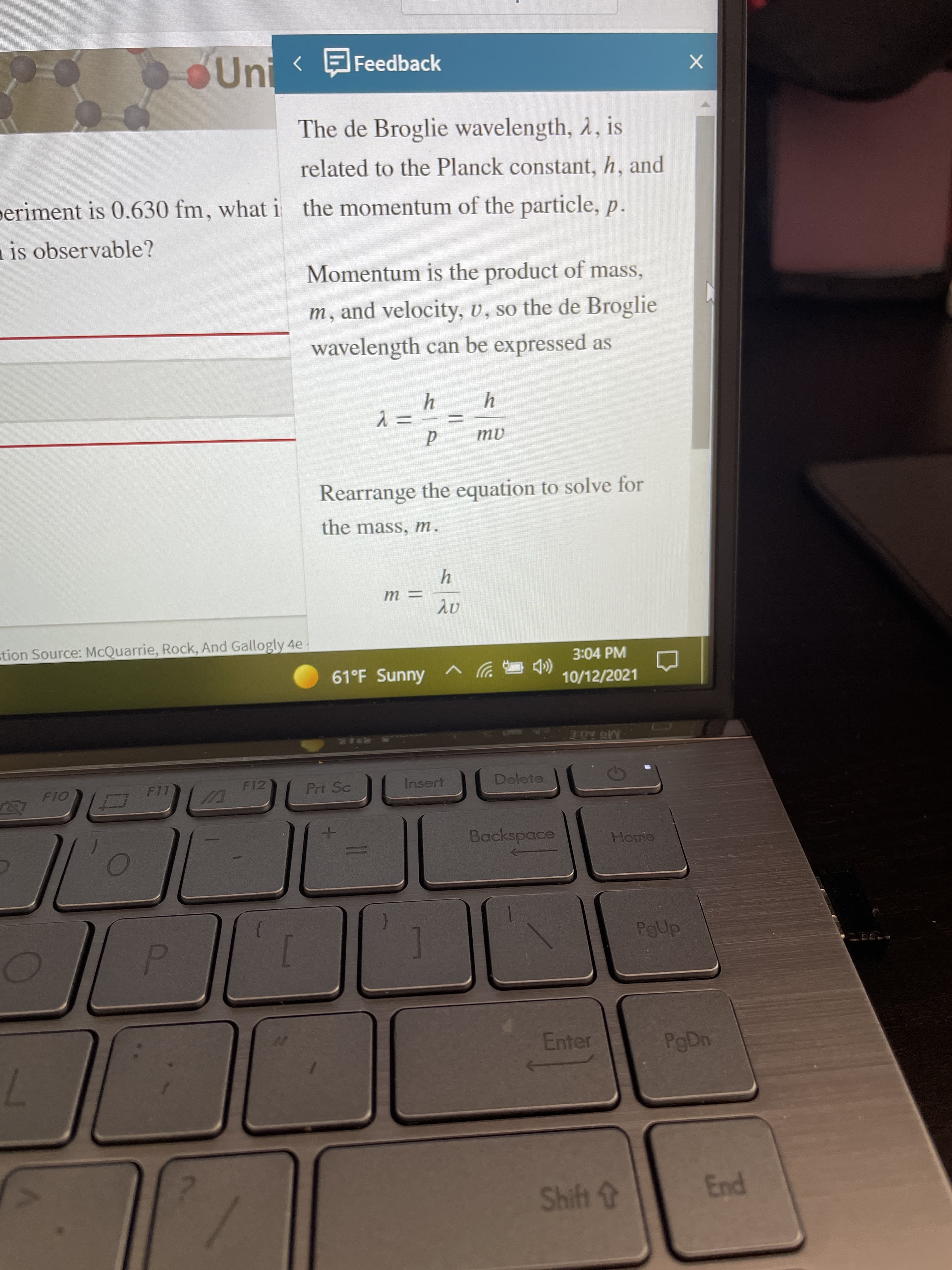
Transcribed Image Text:SUni Feedback
The de Broglie wavelength, 2, is
related to the Planck constant, h, and
periment is 0.630 fm, what i the momentum of the particle, p.
is observable?
Momentum is the product of mass,
m, and velocity, v, so the de Broglie
wavelength can be expressed as
Rearrange the equation to solve for
the mass, m.
stion Source: McQuarrie, Rock, And Gallogly 4e
3:04 PM
61°F Sunny ^ Ca
10/12/2021
F11
F12
Prt Sc
Insert
Delete
Backspace
Home
->
Enter
Shift
pug
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning