Assuming the reaction goes to completion, how many moles of strontium fluoride should we expect to produce ? Reaction How many moles of fluoride ion will be left over once the reaction goes to completion ? Sr(NO3)"+ 2NQ F ce9) ca9) (S)
Assuming the reaction goes to completion, how many moles of strontium fluoride should we expect to produce ? Reaction How many moles of fluoride ion will be left over once the reaction goes to completion ? Sr(NO3)"+ 2NQ F ce9) ca9) (S)
Chapter7: Solutions And Colloids
Section: Chapter Questions
Problem 7.107E
Related questions
Question

Transcribed Image Text:A volume of 100.0 mL of 3.016 M strontium nitrate was added to
200.0 mL of a 3.644 M solution of sodium fluoride. For all problems,
the temperature is held constant at 25 °C.
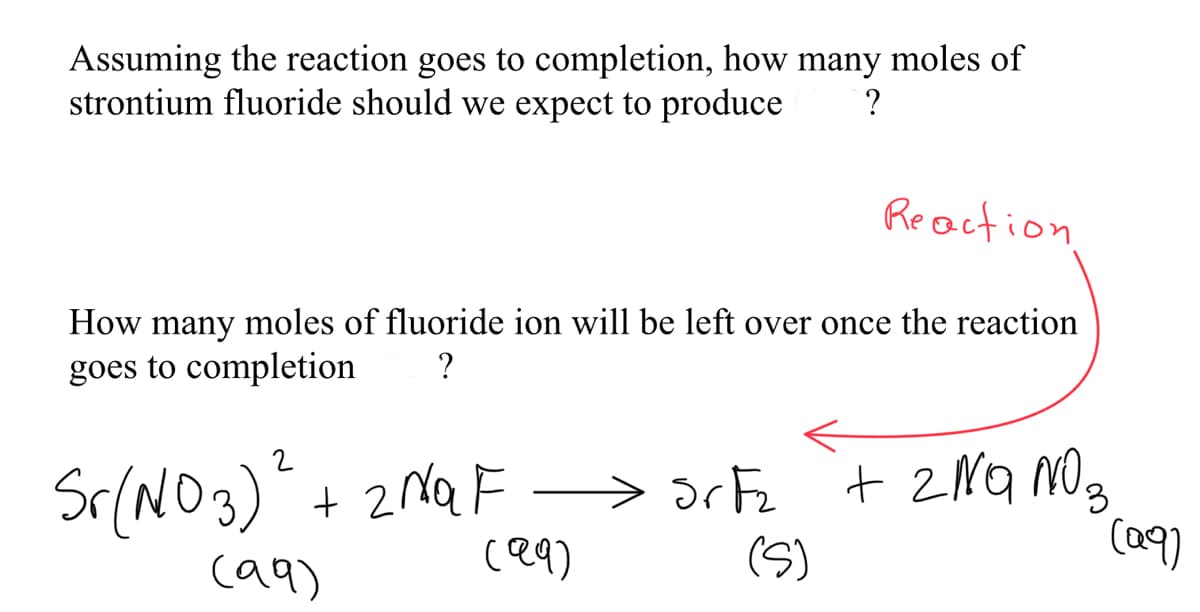
Transcribed Image Text:Assuming the reaction goes to completion, how many moles of
strontium fluoride should we expect to produce
Reaction
How many moles of fluoride ion will be left over once the reaction
goes to completion
?
Sr(NO3)" + 2MQF
ce9)
> 3rFz
(S)
+ 2NQ NO3
(a9)
ca9)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning