At 0 °C, fewer than half the molecules move at speeds Ump = the speed exhibited by the largest At 100 °C, more than Uay = the average (mean) speed of all Urms = the speed of a molecule whose kinetic half the molecules number of molecules the molecules move at speeds greater than 500 m/s. energy is equal to the average (mean) kinetic energy of all the molecules greater than 500 m/s. 0°C 100 °C || 5 x 102 5 x 102 Molecular speed (m/s) 10 x 102 10 x 102 Molecular speed (m/s) (a) (b) A Figure 10.12 Distribution of molecular speed. The relative area under the curve for a range of speeds gives the relative fraction of molecules that have those speeds. (b) Position of most probable (Ump), average (uav), and root- mean-square (urms) speeds of gas molecules. The data shown here are for nitrogen gas at 0 °C. cular speeds for nitrogen gas. (a) The effect of temperature on Activate VWindows Fraction of molecules Fraction of molecules
At 0 °C, fewer than half the molecules move at speeds Ump = the speed exhibited by the largest At 100 °C, more than Uay = the average (mean) speed of all Urms = the speed of a molecule whose kinetic half the molecules number of molecules the molecules move at speeds greater than 500 m/s. energy is equal to the average (mean) kinetic energy of all the molecules greater than 500 m/s. 0°C 100 °C || 5 x 102 5 x 102 Molecular speed (m/s) 10 x 102 10 x 102 Molecular speed (m/s) (a) (b) A Figure 10.12 Distribution of molecular speed. The relative area under the curve for a range of speeds gives the relative fraction of molecules that have those speeds. (b) Position of most probable (Ump), average (uav), and root- mean-square (urms) speeds of gas molecules. The data shown here are for nitrogen gas at 0 °C. cular speeds for nitrogen gas. (a) The effect of temperature on Activate VWindows Fraction of molecules Fraction of molecules
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 130AE
Related questions
Question
Estimate the fraction of molecules at 100 °C with speeds less than 300 m>s.
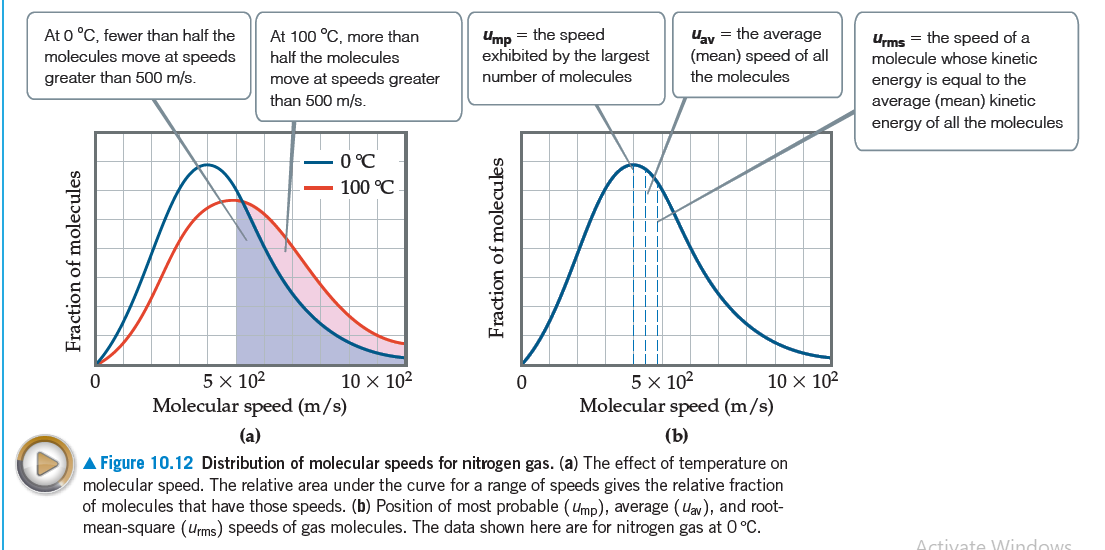
Transcribed Image Text:At 0 °C, fewer than half the
molecules move at speeds
Ump = the speed
exhibited by the largest
At 100 °C, more than
Uay = the average
(mean) speed of all
Urms = the speed of a
molecule whose kinetic
half the molecules
number of molecules
the molecules
move at speeds greater
than 500 m/s.
energy is equal to the
average (mean) kinetic
energy of all the molecules
greater than 500 m/s.
0°C
100 °C
||
5 x 102
5 x 102
Molecular speed (m/s)
10 x 102
10 x 102
Molecular speed (m/s)
(a)
(b)
A Figure 10.12 Distribution of
molecular speed. The relative area under the curve for a range of speeds gives the relative fraction
of molecules that have those speeds. (b) Position of most probable (Ump), average (uav), and root-
mean-square (urms) speeds of gas molecules. The data shown here are for nitrogen gas at 0 °C.
cular speeds for nitrogen gas. (a) The effect of temperature on
Activate VWindows
Fraction of molecules
Fraction of molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax