At 20°C (approximately room temperature) the average velocity of N₂ molecules in air is 1050 mph Part C Y K Part B 1957 ΑΣΦ What is the kinetic energy (in J) of an N₂ molecule moving at this speed? Express your answer in joules to four significant figures. K= Submit ΤΗΣ ΑΣΦ Request Answer Express your answer in joules per mole to four significant figures. What is the total kinetic energy of 1 mol of N₂ molecules moving at this speed? 154894 ? ? J J/mol
At 20°C (approximately room temperature) the average velocity of N₂ molecules in air is 1050 mph Part C Y K Part B 1957 ΑΣΦ What is the kinetic energy (in J) of an N₂ molecule moving at this speed? Express your answer in joules to four significant figures. K= Submit ΤΗΣ ΑΣΦ Request Answer Express your answer in joules per mole to four significant figures. What is the total kinetic energy of 1 mol of N₂ molecules moving at this speed? 154894 ? ? J J/mol
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter3: Measurement And Chemical Calculations
Section: Chapter Questions
Problem 98E
Related questions
Question
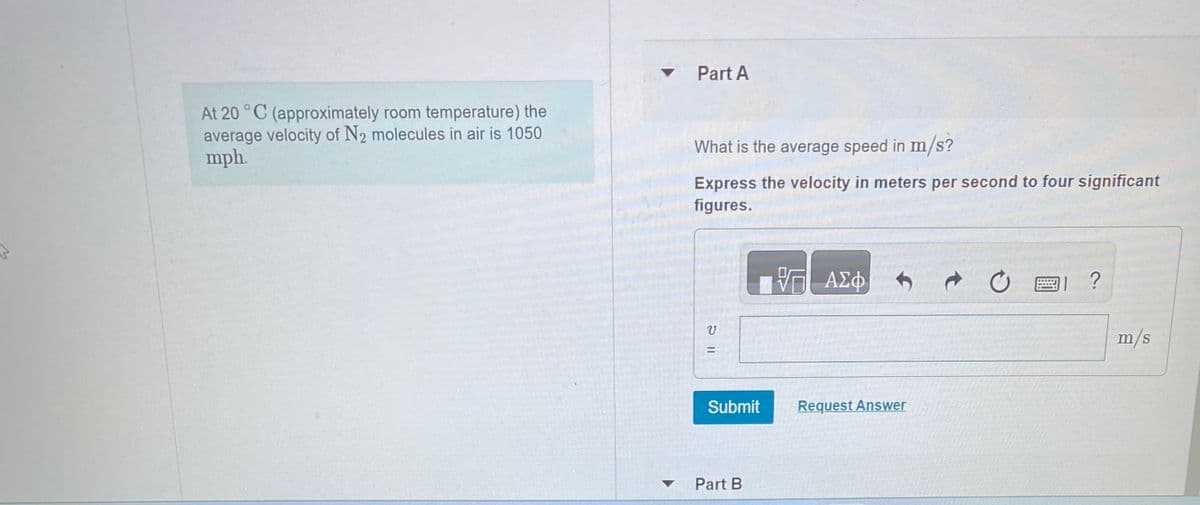
Transcribed Image Text:At 20 °C (approximately room temperature) the
average velocity of N₂ molecules in air is 1050
mph.
▾ Part A
▼
What is the average speed in m/s?
Express the velocity in meters per second to four significant
figures.
V
11 2
=
Submit
Part B
— ΑΣΦ
Request Answer
?
m/s
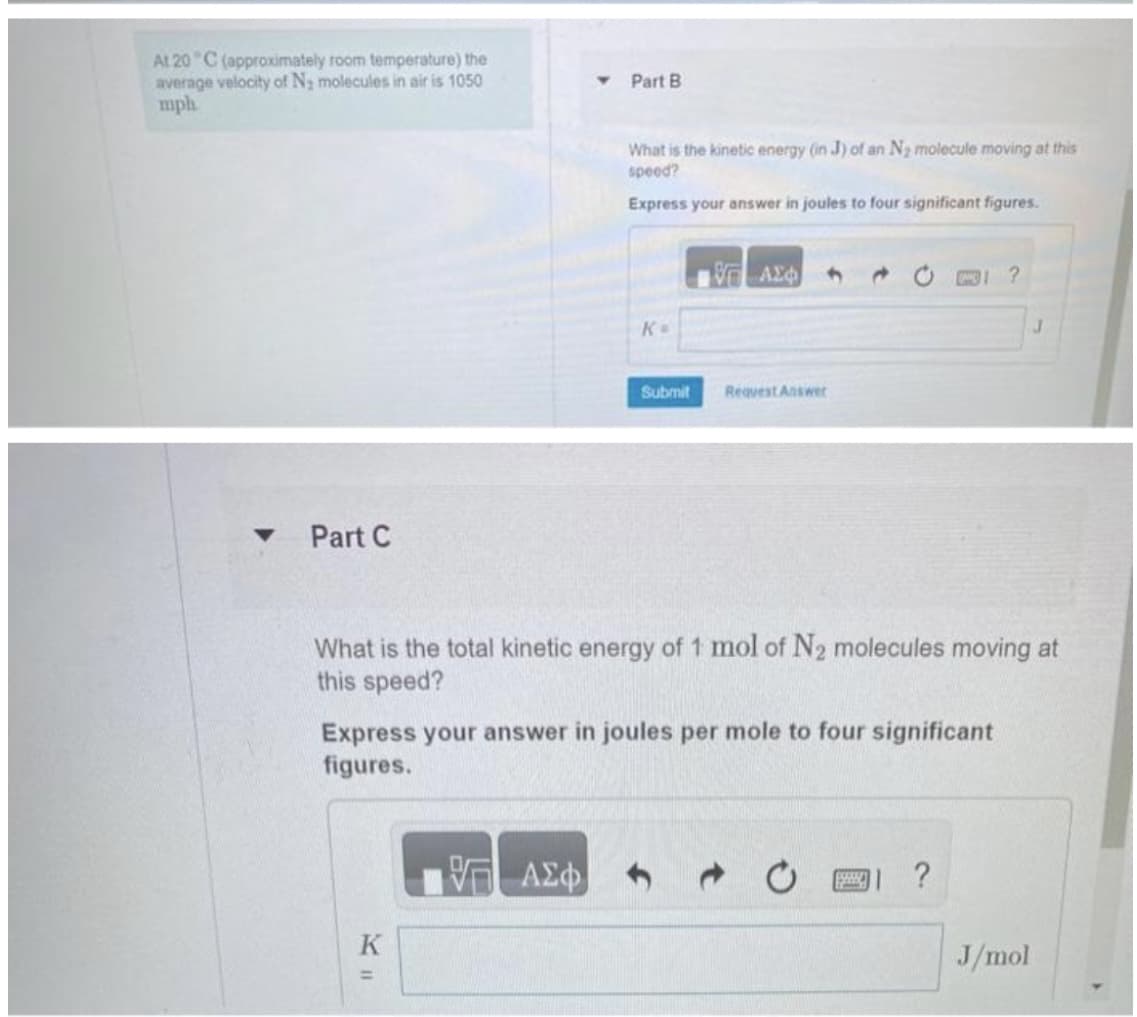
Transcribed Image Text:At 20°C (approximately room temperature) the
average velocity of N₂ molecules in air is 1050
mph
Part C
Part B
K
What is the kinetic energy (in J) of an N₂ molecule moving at this
speed?
Express your answer in joules to four significant figures.
ΑΣΦΙ
K=
Submit
17 ΑΣΦ
Request Answer
Express your answer in joules per mole to four significant
figures.
What is the total kinetic energy of 1 mol of N2₂ molecules moving at
this speed?
www.
?
?
J
J/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning