At -8.64 °C the concentration equilibrium constant K = 7.7 for a certain reaction. Here are some facts about the reaction: -1 • The initial rate of the reaction is 5.3 mol·L¯¹·s¯¹. . If the reaction is run at constant pressure, 132. kJ/mol of heat are absorbed. • The constant pressure molar heat capacity C = 2.97 J'mol K¹. Yes. Using these facts, can you calculate K at 16. °C? x10 O No. If you said yes, then enter your answer at right. Round it to 2 significant digits. If you said no, can you at least decide whether Kat 16. °C will be bigger or smaller than K at -8.64 °C? C Yes, and K will be bigger. Yes, and K will be smaller. No.
At -8.64 °C the concentration equilibrium constant K = 7.7 for a certain reaction. Here are some facts about the reaction: -1 • The initial rate of the reaction is 5.3 mol·L¯¹·s¯¹. . If the reaction is run at constant pressure, 132. kJ/mol of heat are absorbed. • The constant pressure molar heat capacity C = 2.97 J'mol K¹. Yes. Using these facts, can you calculate K at 16. °C? x10 O No. If you said yes, then enter your answer at right. Round it to 2 significant digits. If you said no, can you at least decide whether Kat 16. °C will be bigger or smaller than K at -8.64 °C? C Yes, and K will be bigger. Yes, and K will be smaller. No.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter18: Principles Of Chemical Reactivity: Entropy And Free Energy
Section: Chapter Questions
Problem 54GQ: A crucial reaction for the production of synthetic fuels is the production of H2 by the reaction of...
Related questions
Question
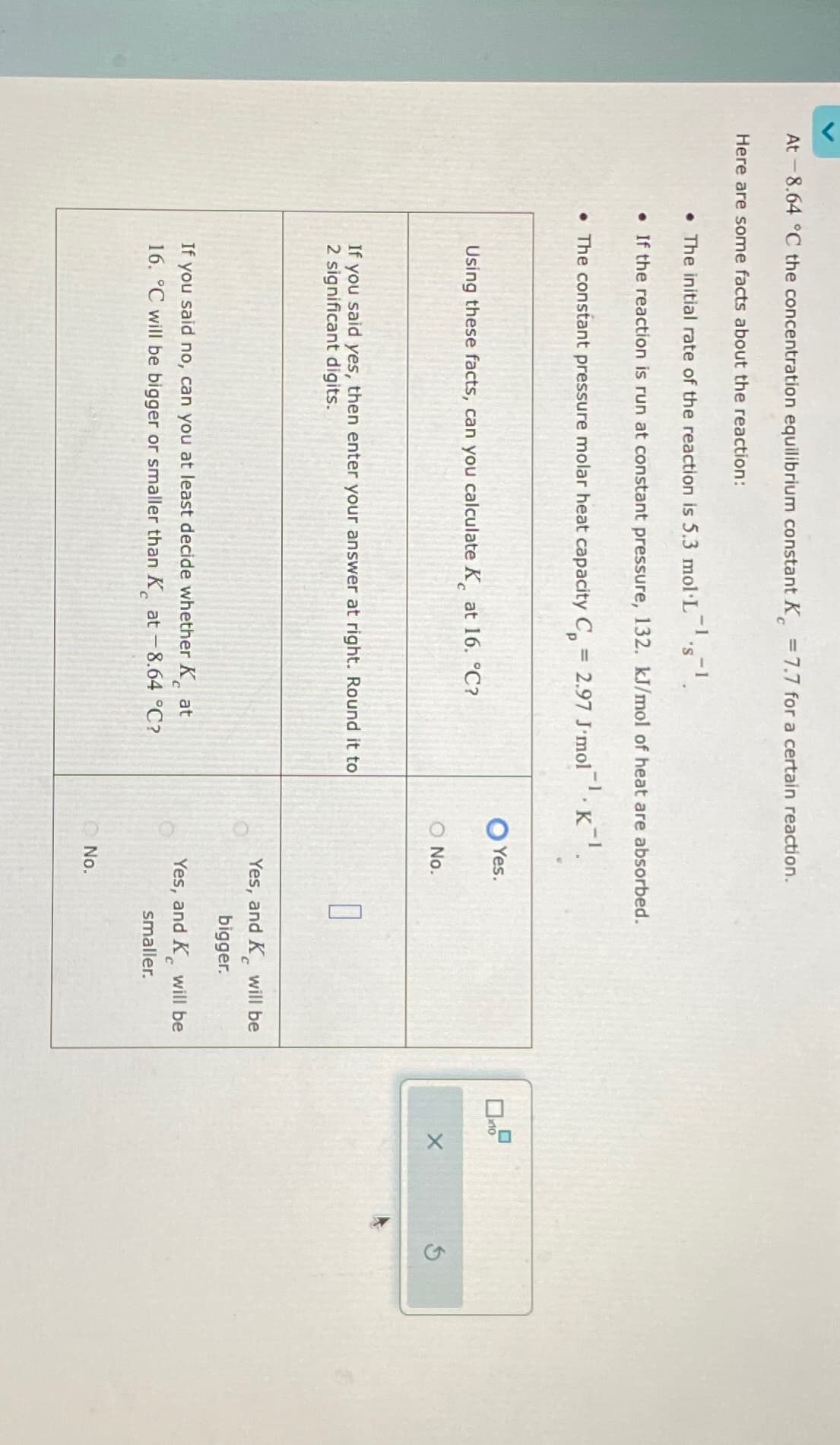
Transcribed Image Text:At -8.64 °C the concentration equilibrium constant K = 7.7 for a certain reaction.
Here are some facts about the reaction:
-1
• The initial rate of the reaction is 5.3 mol·L¯¹·s¯¹.
. If the reaction is run at constant pressure, 132. kJ/mol of heat are absorbed.
• The constant pressure molar heat capacity C =
2.97 J'mol K¹.
Yes.
Using these facts, can you calculate K at 16. °C?
x10
O No.
If you said yes, then enter your answer at right. Round it to
2 significant digits.
If you said no, can you at least decide whether Kat
16. °C will be bigger or smaller than K at -8.64 °C?
C
Yes, and K will be
bigger.
Yes, and K will be
smaller.
No.
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning