At a particular temperature, Kp = 0.27ó for the reaction %3D N204(9) = 2NO2(g) A flask containing only N204(g) at an initial pressure of 3.60 atm is allowed to reach equilibrium. Calculate the total pressure in this flask at equilibrium. 4.060 atm You are correct. Previous Tries Your receipt no. is 159-9433 With no change in the amount of material in the flask, the volume of the container in question is decreased to 0.200 times the original volume. Assuming constant temperature, calculate the (new) total pressure, at equilibrium.
At a particular temperature, Kp = 0.27ó for the reaction %3D N204(9) = 2NO2(g) A flask containing only N204(g) at an initial pressure of 3.60 atm is allowed to reach equilibrium. Calculate the total pressure in this flask at equilibrium. 4.060 atm You are correct. Previous Tries Your receipt no. is 159-9433 With no change in the amount of material in the flask, the volume of the container in question is decreased to 0.200 times the original volume. Assuming constant temperature, calculate the (new) total pressure, at equilibrium.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.116QP
Related questions
Question
100%
ICE chart to calculate value of k
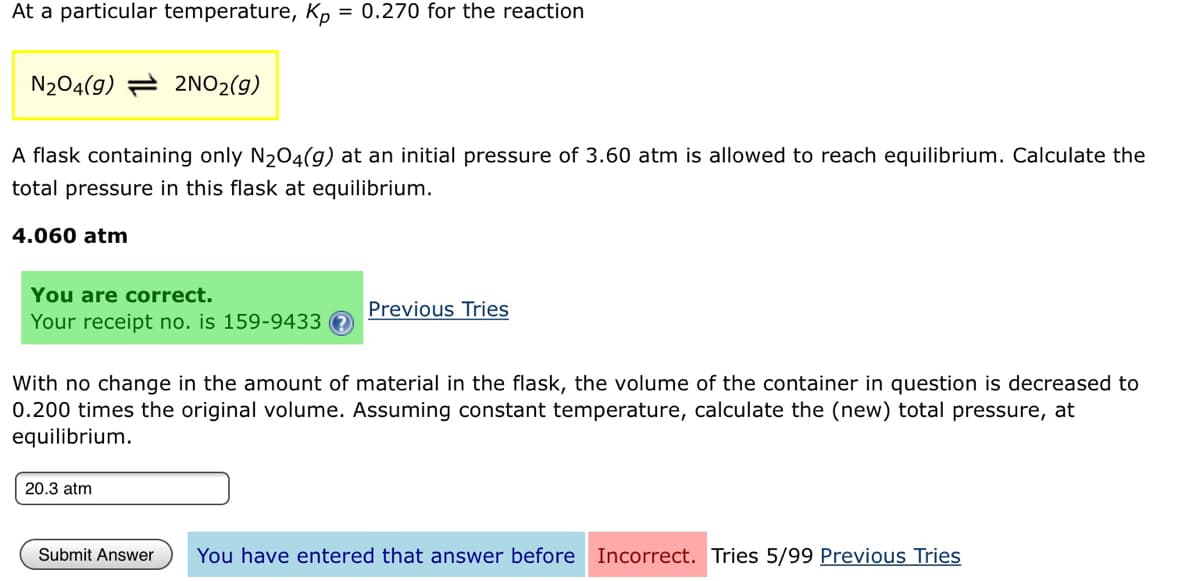
Transcribed Image Text:At a particular temperature, Kp = 0.270 for the reaction
N204(g) = 2NO2(g)
A flask containing only N204(g) at an initial pressure of 3.60 atm is allowed to reach equilibrium. Calculate the
total pressure in this flask at equilibrium.
4.060 atm
You are correct.
Previous Tries
Your receipt no. is 159-9433
With no change in the amount of material in the flask, the volume of the container in question is decreased to
0.200 times the original volume. Assuming constant temperature, calculate the (new) total pressure, at
equilibrium.
20.3 atm
Submit Answer
You have entered that answer before Incorrect. Tries 5/99 Previous Tries
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning