Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section14.6: Molecular Structure And Acid Strength
Problem 14.16CE
Related questions
Question
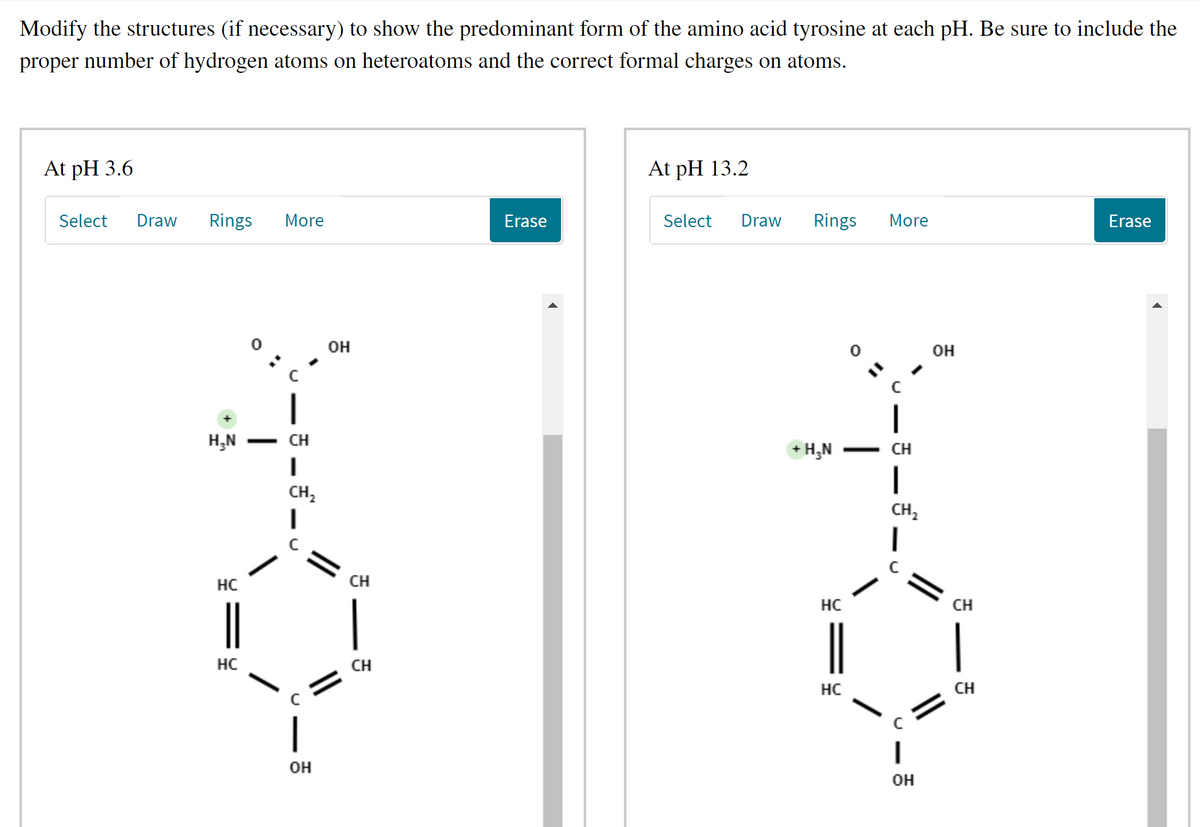
Transcribed Image Text:Modify the structures (if necessary) to show the predominant form of the amino acid tyrosine at each pH. Be sure to include the
proper number of hydrogen atoms on heteroatoms and the correct formal charges on atoms.
At pH 3.6
At pH 13.2
Select
Draw
Rings
More
Erase
Select
Draw
Rings
More
Erase
он
он
H,N
CH
+ H,N
CH
CH,
CH,
HC
CH
HC
CH
|
HC
CH
HC
CH
|
он
Он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning