Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 10RQ: The only stress (change) that also changes the value of K is a change in temperature. For an...
Related questions
Question
100%
i hope that you can answer both the questions. thank you so much!
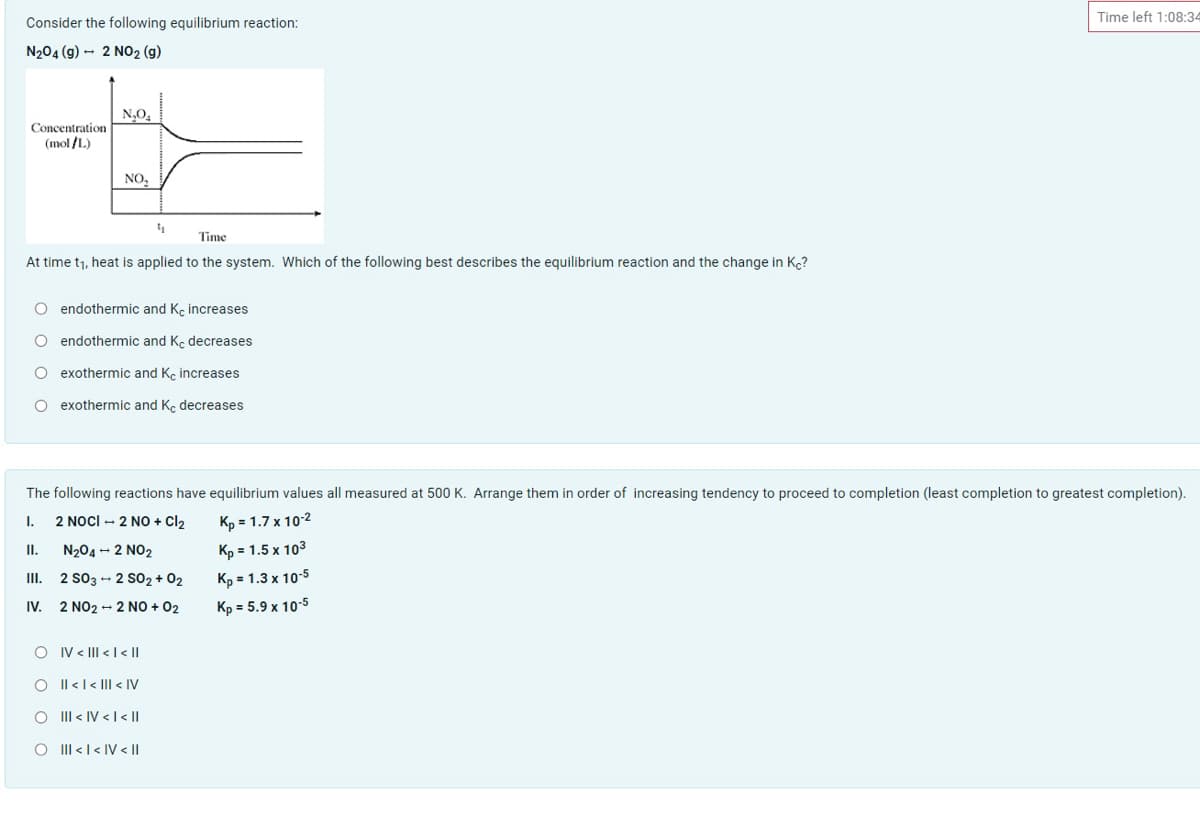
Transcribed Image Text:Time left 1:08:34
Consider the following equilibrium reaction:
N204 (g) - 2 NO2 (g)
N,O,
Concentration
(mol /L)
NO,
Time
At time t1, heat is applied to the system. Which of the following best describes the equilibrium reaction and the change in Kc?
O endothermic and K, increases
O endothermic and Ke decreases
O exothermic and Ke increases
O exothermic and Kç decreases
The following reactions have equilibrium values all measured at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion to greatest completion).
2 NocI - 2 NO + Cl2
Kp = 1.7 x 10-2
I.
II.
N204 - 2 NO2
Kp = 1.5 x 103
III. 2 S03 - 2 sO2 + 02
Kp = 1.3 x 10-5
IV.
2 NO2 - 2 NO + 02
Kp = 5.9 x 10-5
O IV < III < |< ||
Il <l< |II < IV
III < IV < I < ||
O II <l< IV < I|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning