At what pH will the concentration of a compound with a pK, 8,4 be 100 times greater in ita acidic fom than in its basic form? Express your answer using one decimal place. pH =7.9 At what pH will 50% of a compound with a pK= 7.6 be in its basic form? Express your answer using one decimal place. ΑΣφ pH = At what pH will the concentration of a compound with a pK 4.6 be 10 times greater in its basic form than in ts ackdic form? Express your answer using one decimal place. pH = Submit Danunet Anen
At what pH will the concentration of a compound with a pK, 8,4 be 100 times greater in ita acidic fom than in its basic form? Express your answer using one decimal place. pH =7.9 At what pH will 50% of a compound with a pK= 7.6 be in its basic form? Express your answer using one decimal place. ΑΣφ pH = At what pH will the concentration of a compound with a pK 4.6 be 10 times greater in its basic form than in ts ackdic form? Express your answer using one decimal place. pH = Submit Danunet Anen
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 38QRT
Related questions
Question
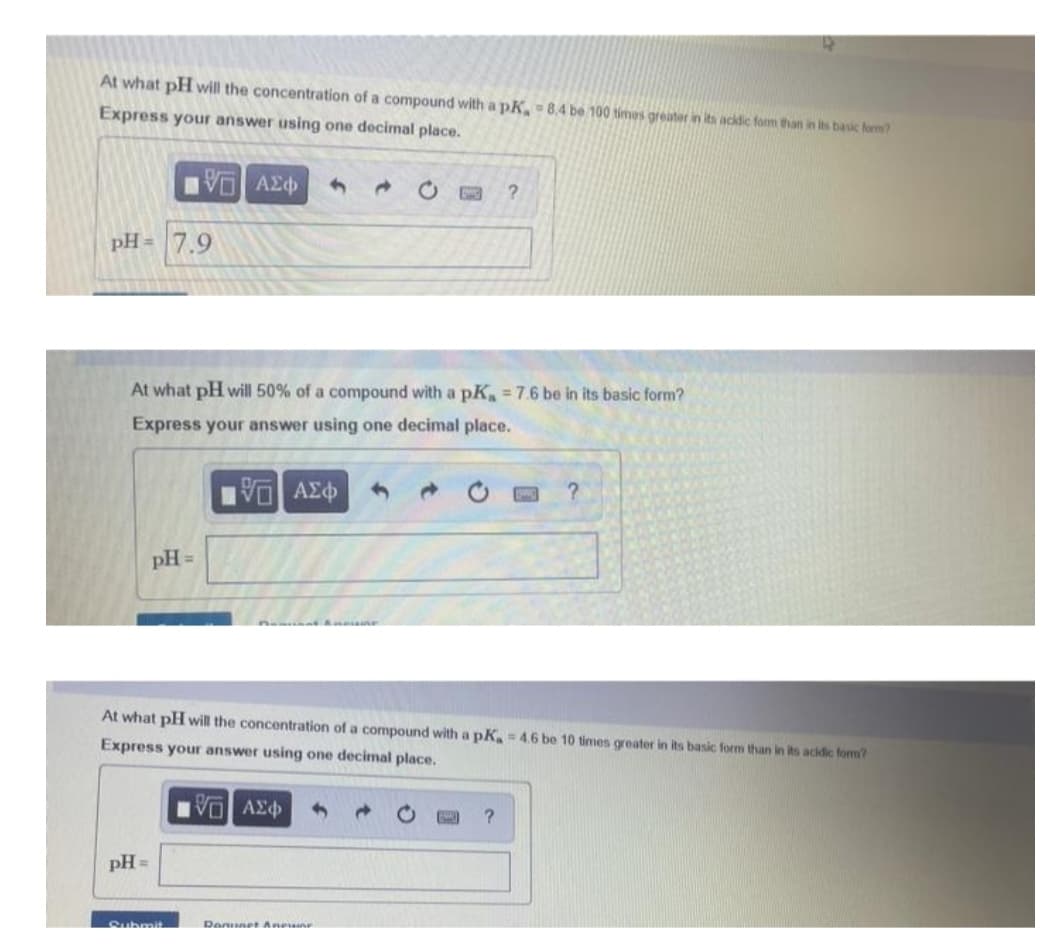
Transcribed Image Text:At what pH will the concentration of a compound with a pK, 8,4 be 100 tímes greater in ita acidic form than in its basic form?
Express your answer using one decimal place.
pH= 7.9
At what pH will 50% of a compound with a pK, = 7.6 be in its basic form?
Express your answer using one decimal place.
pH =
At what pH will the concentration of a compound with a pK = 4.6 be 10 times greater in its basic form than in its acidic form?
Express your answer using one decimal place.
pH =
Submit
Danunet Anenr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning