ated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils are presented below. H₂C b. C. H₂C. H₂C CH₂ H CH CH₂ CH₂ Compound A MM: 136.228 g/mol H₂C CH₂ H H₂ C H₂ CH₂ CH C Hh₂₂₂ Compound B MM: 168.270 g/mol HC HC H₂C CH3 CH CH CH3 OH Compound C MM: 150.212 g/mol 1. Arrange the given compounds in terms of increasing boiling points. 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. a. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? What physical property is the basis of the answer? I Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent.
ated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils are presented below. H₂C b. C. H₂C. H₂C CH₂ H CH CH₂ CH₂ Compound A MM: 136.228 g/mol H₂C CH₂ H H₂ C H₂ CH₂ CH C Hh₂₂₂ Compound B MM: 168.270 g/mol HC HC H₂C CH3 CH CH CH3 OH Compound C MM: 150.212 g/mol 1. Arrange the given compounds in terms of increasing boiling points. 2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature. a. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of liquid remaining in the container? What physical property is the basis of the answer? I Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.89PAE: 89 A number of compounds containing the heavier noble gases, and especially xenon, have been...
Related questions
Question
Answer number 2
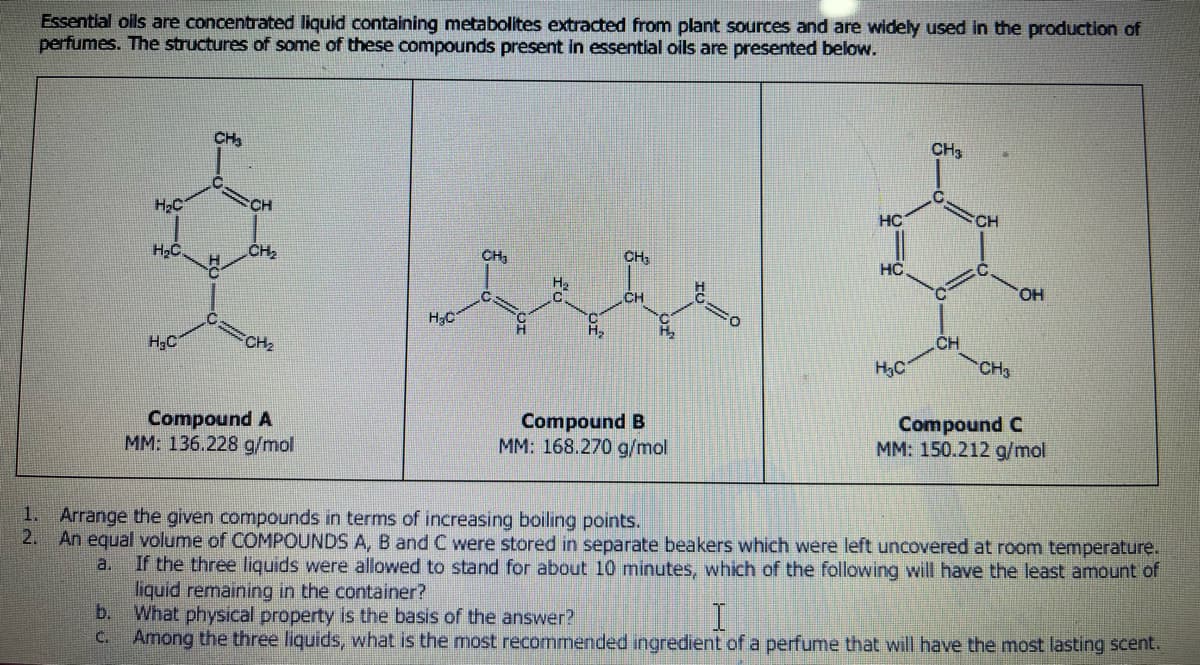
Transcribed Image Text:Essential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of
perfumes. The structures of some of these compounds present in essential oils are presented below.
H₂C
H₂C.
H₂C
CH₂
H
CH
CH₂
CH₂
Compound A
MM: 136.228 g/mol
H₂C
CH₂
H
H₂
H₂
CH₂
CH
Compound B
MM: 168.270 g/mol
HC
HC
H₂C
CH3
C
CH
CH
CH3
OH
Compound C
MM: 150.212 g/mol
1.
Arrange the given compounds in terms of increasing boiling points.
2. An equal volume of COMPOUNDS A, B and C were stored in separate beakers which were left uncovered at room temperature.
a. If the three liquids were allowed to stand for about 10 minutes, which of the following will have the least amount of
liquid remaining in the container?
b.
What physical property is the basis of the answer?
I
c. Among the three liquids, what is the most recommended ingredient of a perfume that will have the most lasting scent.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning