Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter3: Biological Macromolecules
Section: Chapter Questions
Problem 23CTQ: Amino acids have the generic structure seen below, where R represents different carbon-based side...
Related questions
Question
Plz asap
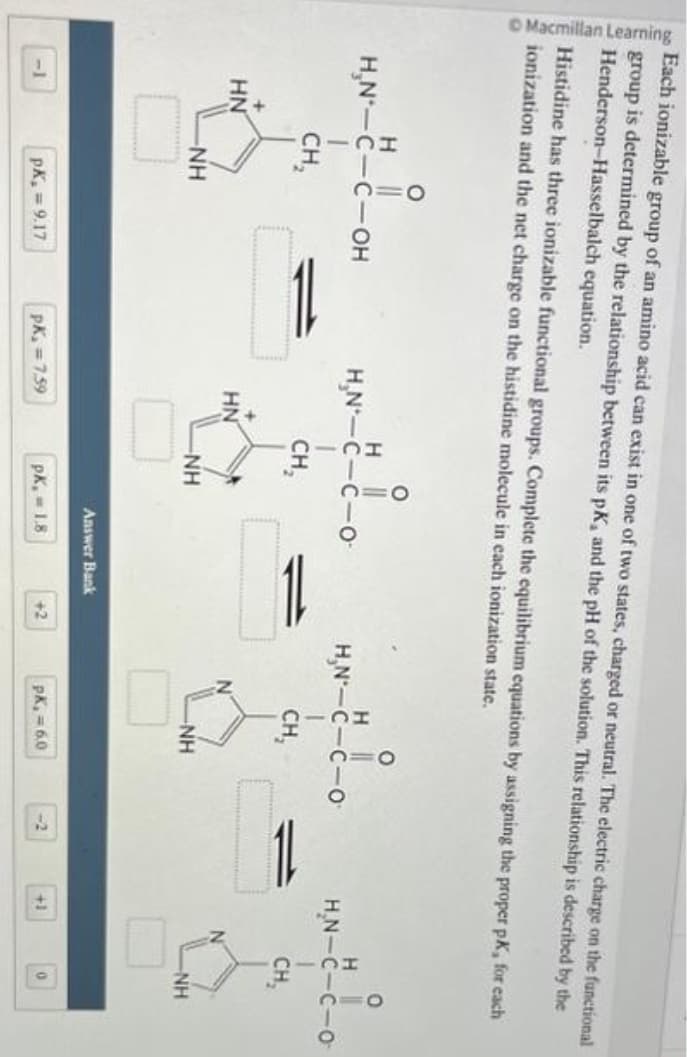
Transcribed Image Text:Macmillan Learning
Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional
group is determined by the relationship between its pK, and the pH of the solution. This relationship is described by the
Henderson-Hasselbalch equation.
Histidine has three ionizable functional groups. Complete the equilibrium equations by assigning the proper pK, for each
ionization and the net charge on the histidine molecule in each ionization state.
O
H ||
H₂N-C-C-OH
CH₂
+
HN
-NH
PK₂=9.17
H
H₂N-C-
HN
PK₁=7.59
CH₂
-NH
-0-
Answer Bank
pk,= 1.8
+2
O
H-L
H₂N-C-C-O
CH₂
-NH
PK, = 6.0
-2
H
H₂N-C-C-O
1
CH₂
+1
-NH
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning