Atomic Wt: O = 15.999 As = 74.922 H = 1.008 Na = 22.990I = 126.904 C = 12.011 1. To balance the equation, what is the coefficient of sodium bicarbonate?* 5 3 2 1 2. What is the total number of electrons gain/loss of arsenic trioxide?* 2 4 6 8 3. What is the molecular weight of the primary standard?* 253.808 g/mol 197.841 g/mol 84.006 g/mol 191.889 g/mol 4. What is the meq of the primary standard? 0.254 0.049 0.099 0.096 5. What is the concentration of the iodine solution? 0.097 N 0.110 N 0.105 N 0.101 N
Atomic Wt: O = 15.999 As = 74.922 H = 1.008 Na = 22.990I = 126.904 C = 12.011 1. To balance the equation, what is the coefficient of sodium bicarbonate?* 5 3 2 1 2. What is the total number of electrons gain/loss of arsenic trioxide?* 2 4 6 8 3. What is the molecular weight of the primary standard?* 253.808 g/mol 197.841 g/mol 84.006 g/mol 191.889 g/mol 4. What is the meq of the primary standard? 0.254 0.049 0.099 0.096 5. What is the concentration of the iodine solution? 0.097 N 0.110 N 0.105 N 0.101 N
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 109AE: Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray...
Related questions
Question
Atomic Wt: O = 15.999 As = 74.922 H = 1.008 Na = 22.990I = 126.904 C = 12.011
1. To balance the equation, what is the coefficient of sodium bicarbonate?*
5
3
2
1
2. What is the total number of electrons gain/loss of arsenic trioxide?*
2
4
6
8
3. What is the molecular weight of the primary standard?*
253.808 g/mol
197.841 g/mol
84.006 g/mol
191.889 g/mol
4. What is the meq of the primary standard?
0.254
0.049
0.099
0.096
5. What is the concentration of the iodine solution?
0.097 N
0.110 N
0.105 N
0.101 N
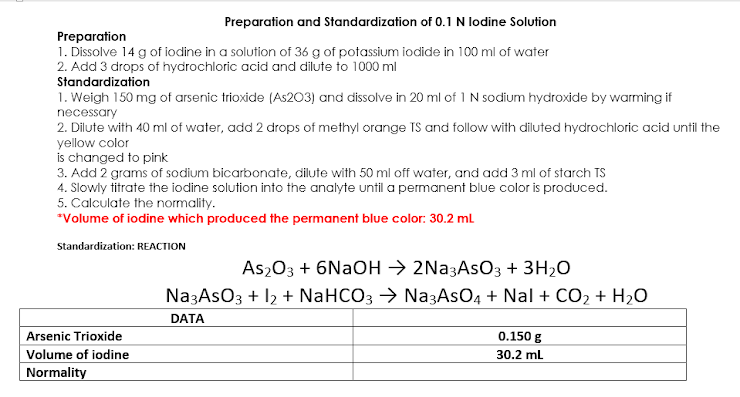
Transcribed Image Text:Preparation and Standardization of 0.1 N lodine Solution
Preparation
1. Dissolve 14 g of iodine in a solution of 36 g of potassium iodide in 100 ml of water
2. Add 3 drops of hydrochloric acid and dilute to 1000 ml
Standardization
1. Weigh 150 mg of arsenic trioxide (As203) and dissolve in 20 ml of 1 N sodium hydroxide by warming if
necessary
2. Dilute with 40 ml of water, add 2 drops of methyl orange TS and follow with diluted hydrochloric acid until the
yellow color
is changed to pink
3. Add 2 grams of sodium bicarbonate, dilute with 50 ml off water, and add 3 ml of starch TS
4. Slowly titrate the iodine solution into the analyte until a permanent blue color is produced.
5. Calculate the normality.
*Volume of iodine which produced the permanent blue color: 30.2 mL
Standardization: REACTION
Arsenic Trioxide
Volume of iodine
Normality
As₂O3 + 6NaOH → 2Na3AsO3 + 3H₂O
Na3ASO3 + 12 + NaHCO3 → Na3AsO4 + Nal + CO₂ + H₂O
DATA
0.150 g
30.2 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
