B) -15.5 E) 40.5 12) Given the data in the table below, AH°rxn for the reaction C) -109 12) D D) –59.0 3NO2 (g) + H2O (I) – 2HNO3 (aq) + NO (g) is kJ. Substance AH°f (kJ/mol) H2O (1) -286 NO (g) NO2 (g) 90 34 HNO3 (aq) -207 NH3 (g) -46 A) 140 E) -508 B) 64 C) -64 D) -140 13) B dupie of calcium carbonate [CaCOa (slabsorbs 40.3 I of heat, upon which the temperature of the e sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-K, what is the mass (in grams) of the sample? A) 5.1 g E) 5.3 g B) 7.6 g C) -7.6 g D) 0.13 g 14) D nic temperature of a 35.1 g sample of iron increases from 24.5 °C to 31.8 °C. If the specific heat of iron is 0.450 J/g-K, how many joules of heat are absorbed? A) 0.722 J E) 3.29 J B) 0.0936 J C) -115 J D) 115 J SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 15) 455S 15) The AHrxn for the combustion of methane is -890.0 kJ. How much heat energy (kJ) is released if 82.1 grams of methane are burned in an excess amount of oxygen? 3 VOodward Stoltzfus • Michael W. Lufaso
B) -15.5 E) 40.5 12) Given the data in the table below, AH°rxn for the reaction C) -109 12) D D) –59.0 3NO2 (g) + H2O (I) – 2HNO3 (aq) + NO (g) is kJ. Substance AH°f (kJ/mol) H2O (1) -286 NO (g) NO2 (g) 90 34 HNO3 (aq) -207 NH3 (g) -46 A) 140 E) -508 B) 64 C) -64 D) -140 13) B dupie of calcium carbonate [CaCOa (slabsorbs 40.3 I of heat, upon which the temperature of the e sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-K, what is the mass (in grams) of the sample? A) 5.1 g E) 5.3 g B) 7.6 g C) -7.6 g D) 0.13 g 14) D nic temperature of a 35.1 g sample of iron increases from 24.5 °C to 31.8 °C. If the specific heat of iron is 0.450 J/g-K, how many joules of heat are absorbed? A) 0.722 J E) 3.29 J B) 0.0936 J C) -115 J D) 115 J SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 15) 455S 15) The AHrxn for the combustion of methane is -890.0 kJ. How much heat energy (kJ) is released if 82.1 grams of methane are burned in an excess amount of oxygen? 3 VOodward Stoltzfus • Michael W. Lufaso
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 89QAP: A wad of steel wool (specific heat=0.45J/gC) at 25C is misplaced in a microwave oven, which later is...
Related questions
Question
Question 15
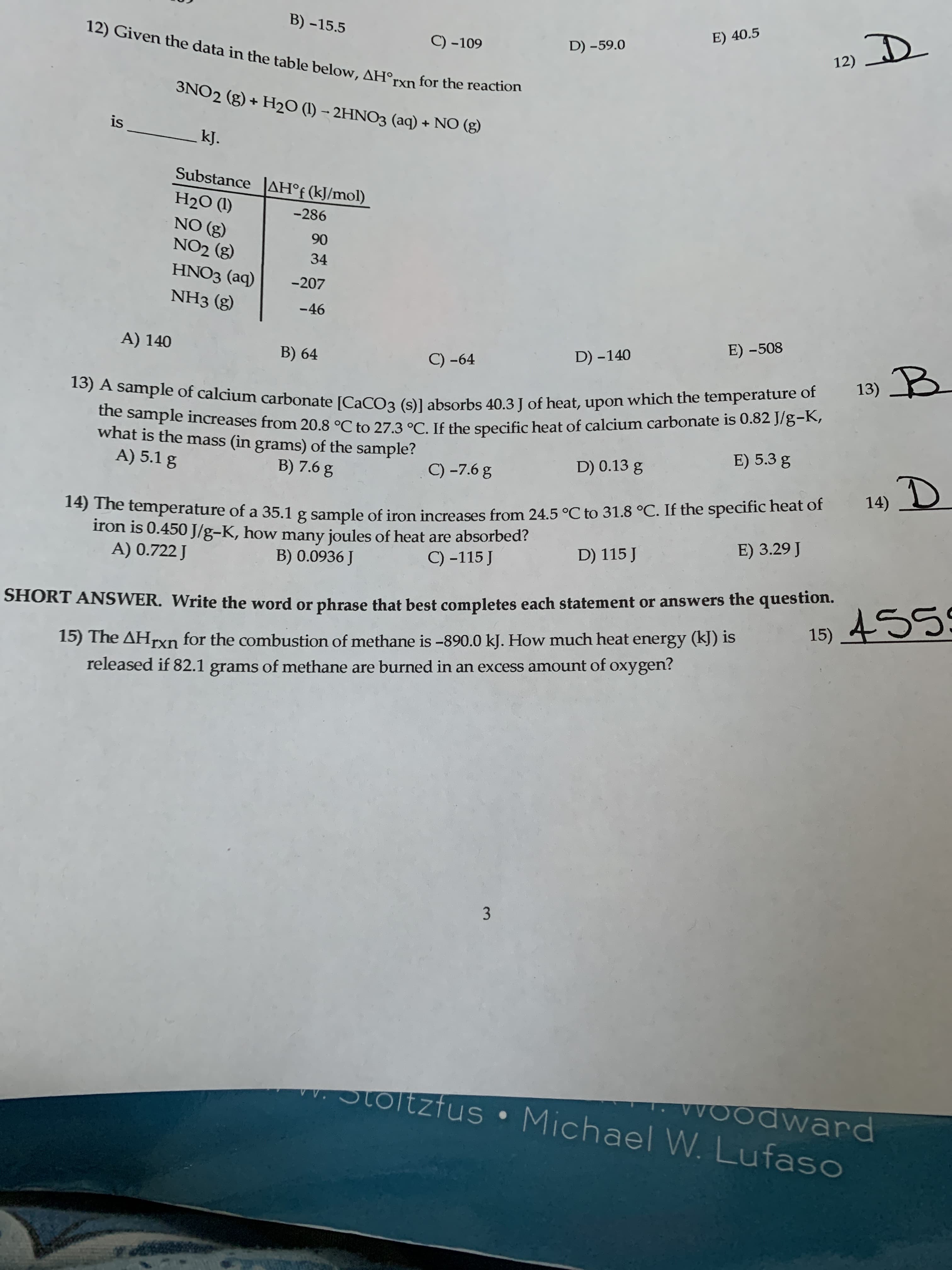
Transcribed Image Text:B) -15.5
E) 40.5
12) Given the data in the table below, AH°rxn for the reaction
C) -109
12) D
D) –59.0
3NO2 (g) + H2O (I) – 2HNO3 (aq) + NO (g)
is
kJ.
Substance AH°f (kJ/mol)
H2O (1)
-286
NO (g)
NO2 (g)
90
34
HNO3 (aq)
-207
NH3 (g)
-46
A) 140
E) -508
B) 64
C) -64
D) -140
13) B
dupie of calcium carbonate [CaCOa (slabsorbs 40.3 I of heat, upon which the temperature of
the
e sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-K,
what is the mass (in grams) of the sample?
A) 5.1 g
E) 5.3 g
B) 7.6 g
C) -7.6 g
D) 0.13 g
14) D
nic temperature of a 35.1 g sample of iron increases from 24.5 °C to 31.8 °C. If the specific heat of
iron is 0.450 J/g-K, how many joules of heat are absorbed?
A) 0.722 J
E) 3.29 J
B) 0.0936 J
C) -115 J
D) 115 J
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
15) 455S
15) The AHrxn for the combustion of methane is -890.0 kJ. How much heat energy (kJ) is
released if 82.1 grams of methane are burned in an excess amount of oxygen?
3
VOodward
Stoltzfus • Michael W. Lufaso
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning