(b) A sample of 4.0 mol O, (g) is originally confined in 20 dm³ at 270 K and then undergoes adiabatic expansion against pressure of 600 Torr until the volume has increased by a factor of 3.0. Calculate q, w, AT, AU and a constant ΔΗ
(b) A sample of 4.0 mol O, (g) is originally confined in 20 dm³ at 270 K and then undergoes adiabatic expansion against pressure of 600 Torr until the volume has increased by a factor of 3.0. Calculate q, w, AT, AU and a constant ΔΗ
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.76E
Related questions
Question
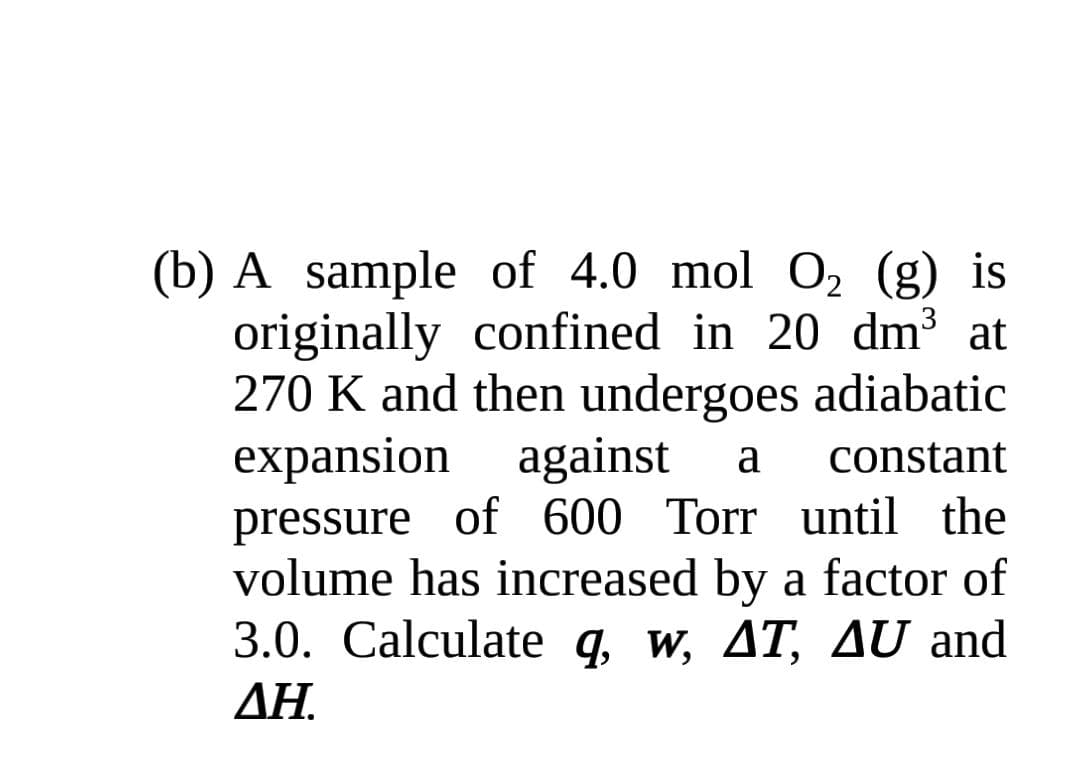
Transcribed Image Text:(b) A sample of 4.0 mol O, (g) is
originally confined in 20 dm3 at
270 K and then undergoes adiabatic
expansion against
pressure of 600 Torr until the
volume has increased by a factor of
3.0. Calculate q, w, AT, AU and
a
constant
ΔΗ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning