B is a weak base with Kp = (4.11x10^-9). What is the percent ionization of a (7.060x10^-1) M solution of B? Enter your answer in scientific notation with 3 sig figs. Do not include any units in your answer. Do not round any intermediate calculations. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer
B is a weak base with Kp = (4.11x10^-9). What is the percent ionization of a (7.060x10^-1) M solution of B? Enter your answer in scientific notation with 3 sig figs. Do not include any units in your answer. Do not round any intermediate calculations. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answer
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 32QRT
Related questions
Question
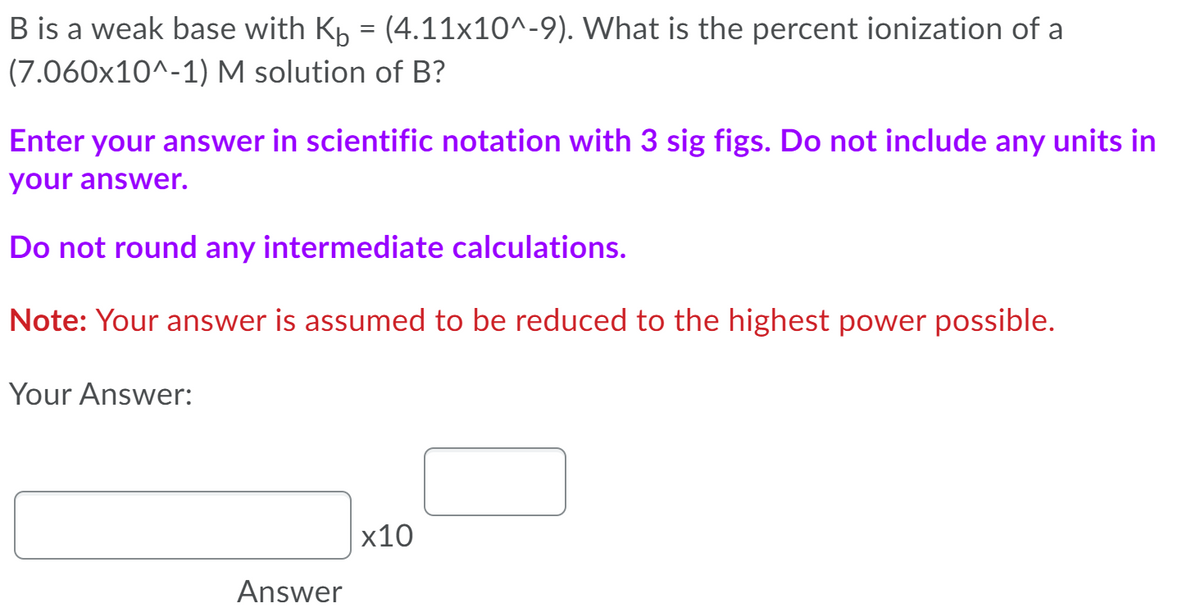
Transcribed Image Text:B is a weak base with Kp = (4.11x10^-9). What is the percent ionization of a
(7.060x10^-1) M solution of B?
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
x10
Answer
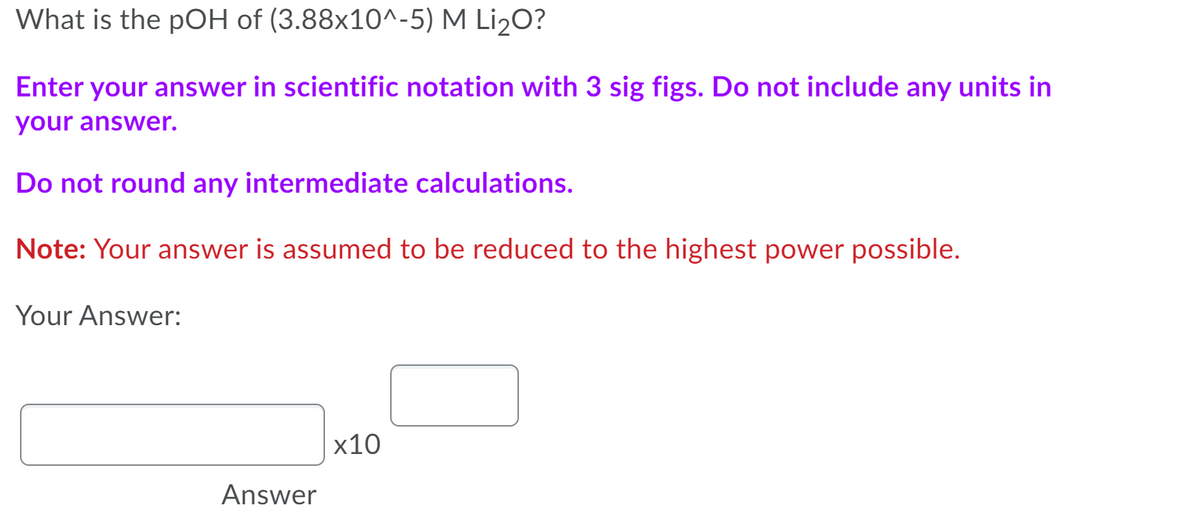
Transcribed Image Text:What is the pOH of (3.88x10^-5) M Li2O?
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
х10
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning