Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter12: Atomic X-ray Spectrometry
Section: Chapter Questions
Problem 12.6QAP
Related questions
Question
Transcribed Image Text:I L Page view
Read aloud
7 Draw
E Hig
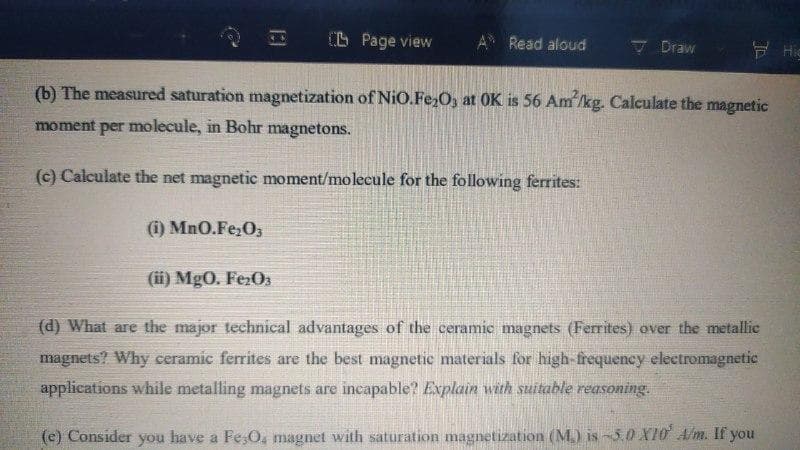
(b) The measured saturation magnetization of NiO.Fe,O, at OK is 56 Am'/kg. Calculate the magnetic
moment per molecule, in Bohr magnetons.
(c) Calculate the net magnetic moment/molecule for the following ferrites:
(i) MnO.Fe;O,
(ii) MgO. Fe:O3
(d) What are the major technical advantages of the ceramic magnets (Ferrites) over the metallic
magnets? Why ceramic ferrites are the best magnetic materials for high-frequency electromagnetic
applications while metalling magnets are incapable? Explain with suitable reasoning.
(e) Consider you have a Fe;O, magnet with saturation magnetization (M.) is-3.0 X10 A/m. If you
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning