(b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral (pH 6-8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose a reasonable mechanism for this reaction. он он pH 7 H,0 LIOH HO co2 он о OH OH OH O 10
(b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral (pH 6-8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose a reasonable mechanism for this reaction. он он pH 7 H,0 LIOH HO co2 он о OH OH OH O 10
Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.SE: Something Extra
Problem 41AP: In light of your answer to Problem 30-40, explain why a mixture of products occurs in the following...
Related questions
Question
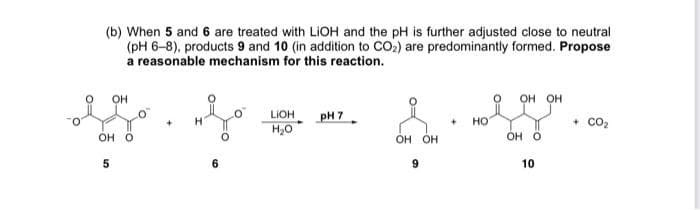
Transcribed Image Text:(b) When 5 and 6 are treated with LIOH and the pH is further adjusted close to neutral
(pH 6-8), products 9 and 10 (in addition to CO2) are predominantly formed. Propose
a reasonable mechanism for this reaction.
OH
он он
pH 7
LIOH
H20
но
co2
Он о
он он
OH O
6.
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

