(b). Atomic abs orption spectroscopy (AAS) is used for the quantitative determination of chemical elements in a sample which is based on absorption of light by free atoms. (i). State the type of element that can be analysed by AAS. (ii). In AAS, an atomic constituent in a sample will be analysed after atomisation process. Explain the principles of atomisation. (iii). Differentiate between a flame atomiser and an electrothermal atomiser, in terms of atomisation mode, temperature used and sensitivity.
(b). Atomic abs orption spectroscopy (AAS) is used for the quantitative determination of chemical elements in a sample which is based on absorption of light by free atoms. (i). State the type of element that can be analysed by AAS. (ii). In AAS, an atomic constituent in a sample will be analysed after atomisation process. Explain the principles of atomisation. (iii). Differentiate between a flame atomiser and an electrothermal atomiser, in terms of atomisation mode, temperature used and sensitivity.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter18: Raman Spectroscopy
Section: Chapter Questions
Problem 18.6QAP
Related questions
Question
kindly answer this question B i, ii & iii
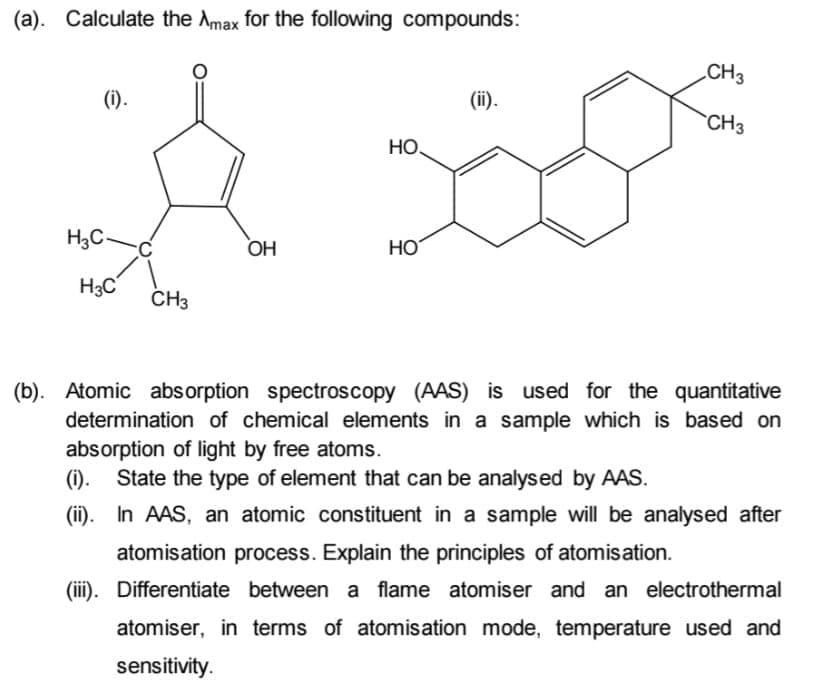
Transcribed Image Text:(a). Calculate the Amax for the following compounds:
CH3
(i).
(ii).
CH3
НО.
H3C
OH
HO
H3C
CH3
(b). Atomic abs orption spectroscopy (AAS) is used for the quantitative
determination of chemical elements in a sample which is based on
absorption of light by free atoms.
(i).
State the type of element that can be analysed by AAS.
(ii). In AAS, an atomic constituent in a sample will be analysed after
atomisation process. Explain the principles of atomisation.
(iii). Differentiate between a flame atomiser and an electrothermal
atomiser, in terms of atomisation mode, temperature used and
sensitivity.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning
