B. Determination of the Molar Mass of an Unknown Acid, HA Chemical Reactions: Molecular Equation: HA(ag) + NaOH(ag) - NaA(ag) + H,0(1) Net Ionic Equation: HA(ag) + OH(ag) -– H,O(1) + A'(ag) Details Trial 1 Trial 2 Trial 3 Mass of container plus contents (g) Mass of container less contents (g) Mass (g) of Unknown sample Obtained Average Molarity of NaOH Initial Buret Reading (mL) Final Buret Reading (mL) Volume (mL) NaOH Delivered No. of mmol NaOH 27.8792 28.7518 28.4300 27.4518 28.3217 28.0013 0.4274 0.4301 0.1156 M 0.50 0.4287 1.20 1.00 19.30 18.67 19.27 x 103 х 103 No. of Moles of Unknown Acid, HA Molar Mass (g/mole) Average Molar Mass (g/mol) Deviation х 103
B. Determination of the Molar Mass of an Unknown Acid, HA Chemical Reactions: Molecular Equation: HA(ag) + NaOH(ag) - NaA(ag) + H,0(1) Net Ionic Equation: HA(ag) + OH(ag) -– H,O(1) + A'(ag) Details Trial 1 Trial 2 Trial 3 Mass of container plus contents (g) Mass of container less contents (g) Mass (g) of Unknown sample Obtained Average Molarity of NaOH Initial Buret Reading (mL) Final Buret Reading (mL) Volume (mL) NaOH Delivered No. of mmol NaOH 27.8792 28.7518 28.4300 27.4518 28.3217 28.0013 0.4274 0.4301 0.1156 M 0.50 0.4287 1.20 1.00 19.30 18.67 19.27 x 103 х 103 No. of Moles of Unknown Acid, HA Molar Mass (g/mole) Average Molar Mass (g/mol) Deviation х 103
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.57PAE
Related questions
Question
exolain how to solve trial one so i can do the remaining trials on my own. thanks
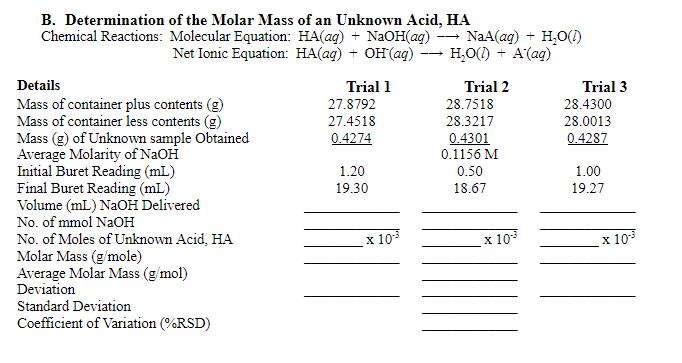
Transcribed Image Text:B. Determination of the Molar Mass of an Unknown Acid, HA
Chemical Reactions: Molecular Equation: HA(ag) + NaOH(ag)
+ H,O()
NaA(ag)
Net Ionic Equation: HA(ag) + OH(ag) -→ H,O(1) + A(ag)
Details
Trial 1
Trial 2
Trial 3
Mass of container plus contents (g)
Mass of container less contents (g)
Mass (g) of Unknown sample Obtained
Average Molarity of NaOH
Initial Buret Reading (mL)
Final Buret Reading (mL)
Volume (mL) NAOH Delivered
27.8792
28.7518
28.4300
27.4518
28.3217
28.0013
0.4274
0.4301
0.1156 M
0.4287
1.20
0.50
1.00
19.30
18.67
19.27
No. of mmol NaOH
х 103
No. of Moles of Unknown Acid, HA
Molar Mass (g/mole)
Average Molar Mass (g/mol)
Deviation
х 103
х 103
Standard Deviation
Coefficient of Variation (%RSD)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning