A Volumetric Analysis A. Standardization of a Sodium Hydroxide Solution Part A.3. Approximate [NaOH] = 0.16 M Part A.4. Approximate mass of KHC,H,O̟ = ~0.49 g %3D Trial 1 Trial 2 Trial 3 1 Tared mass of KHC,H,O, (g) 2 Molar mass of KHC,H,O, (g/mol) 3 Moles of KHC,H,O, (mol) 4 Buret Reading of NaOH, initial (mL) 5 Buret Reading of NaOH, fhal (mL) 6 Volume of NAOH dispensed (mL) 7 Molar Concentration of NAOH (mol/L) 8 Average molar concentration of NaOH (mol/L) 9 Standard deviation 10 Relative standard deviation (%RSD) 5.00E-01 5.50E-01 5.80E-01 (~0.5 g) 204.44 50.00 35.00 50.00 34.00 50.00 36.00 (~0.16 M)
A Volumetric Analysis A. Standardization of a Sodium Hydroxide Solution Part A.3. Approximate [NaOH] = 0.16 M Part A.4. Approximate mass of KHC,H,O̟ = ~0.49 g %3D Trial 1 Trial 2 Trial 3 1 Tared mass of KHC,H,O, (g) 2 Molar mass of KHC,H,O, (g/mol) 3 Moles of KHC,H,O, (mol) 4 Buret Reading of NaOH, initial (mL) 5 Buret Reading of NaOH, fhal (mL) 6 Volume of NAOH dispensed (mL) 7 Molar Concentration of NAOH (mol/L) 8 Average molar concentration of NaOH (mol/L) 9 Standard deviation 10 Relative standard deviation (%RSD) 5.00E-01 5.50E-01 5.80E-01 (~0.5 g) 204.44 50.00 35.00 50.00 34.00 50.00 36.00 (~0.16 M)
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.23QAP
Related questions
Question
![Lecture 6 Titration (1) - Compatibility Mode - Saved to this PC
Danielle
Draw
Design
Layout
References
Mailings
Review
View
Help
O Search
O Find
Replace
A A Aa A
E- EE EE E ¶
AAB6CCL AaBbCeI AaBbCcI AaBbC AaBbC
ab x, x A Av
三=三、、田。
1 Caption
1 Normal
1 No Spac... Heading 1
Title
A Select v
Font
Paragraph
Styles
Editing
Lecture 6: Titration
Name:
A Volumetric Analysis
A. Standardization of a Sodium Hydroxide Solution
Part A.3. Approximate [NaOH] = 0.16 M
Part A.4. Apprdximate mass of KHC,H,O, = ~0.49 g
Trial 1
Trial 2
Trial 3
1 Tared mass of KHC,H,O, (g)
2 Molar mass of KHC,H,0, (g/mol)
3 Moles of KHC,H,O, (mol)
4 Buret Reading of NaOH, initial (mL)
5 Buret Reading of NaOH, f hal (mL)
6 Volume of NaOH dispensed (mL)
7 Molar Concentration of NAOH (mol/L)
8 Average molar concentration of NaOH (molL)
9 Standard deviation
10 Relative standard deviation (%RSD)
5.00E-01
5.50E-01
5.80E-01
(~0.5 g)
204.44
50.00
35.00
50.00
34.00
50.00
36.00
(~0.16 M)
B. Molar Concentration of an Acid Solution
Acid type (enter "1" for monoprotic and "2" for diprotic)
Unknown No.
1
3
Sample 2
Samalo 2
Cample1
D Focus
A Rain to stop
to search
DAL
prt sc
F10
home
F1
end
F12
CQ
F4
&
%23
7
08.
3.
U
W
G
K
LLI](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc82da06e-ae09-42a6-970d-e61b6c80277c%2F0f66f14c-a157-4190-9018-cc0c59d72454%2Fdnqowfk_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Lecture 6 Titration (1) - Compatibility Mode - Saved to this PC
Danielle
Draw
Design
Layout
References
Mailings
Review
View
Help
O Search
O Find
Replace
A A Aa A
E- EE EE E ¶
AAB6CCL AaBbCeI AaBbCcI AaBbC AaBbC
ab x, x A Av
三=三、、田。
1 Caption
1 Normal
1 No Spac... Heading 1
Title
A Select v
Font
Paragraph
Styles
Editing
Lecture 6: Titration
Name:
A Volumetric Analysis
A. Standardization of a Sodium Hydroxide Solution
Part A.3. Approximate [NaOH] = 0.16 M
Part A.4. Apprdximate mass of KHC,H,O, = ~0.49 g
Trial 1
Trial 2
Trial 3
1 Tared mass of KHC,H,O, (g)
2 Molar mass of KHC,H,0, (g/mol)
3 Moles of KHC,H,O, (mol)
4 Buret Reading of NaOH, initial (mL)
5 Buret Reading of NaOH, f hal (mL)
6 Volume of NaOH dispensed (mL)
7 Molar Concentration of NAOH (mol/L)
8 Average molar concentration of NaOH (molL)
9 Standard deviation
10 Relative standard deviation (%RSD)
5.00E-01
5.50E-01
5.80E-01
(~0.5 g)
204.44
50.00
35.00
50.00
34.00
50.00
36.00
(~0.16 M)
B. Molar Concentration of an Acid Solution
Acid type (enter "1" for monoprotic and "2" for diprotic)
Unknown No.
1
3
Sample 2
Samalo 2
Cample1
D Focus
A Rain to stop
to search
DAL
prt sc
F10
home
F1
end
F12
CQ
F4
&
%23
7
08.
3.
U
W
G
K
LLI
Transcribed Image Text:A A Aa A
AaBbCcL AaBbCcI AaBbCcI AaBbC AaBbC
A P. A.
1 Caption
1 Normal
1 No Spac. Heading 1
Title
Paragraph
Styles
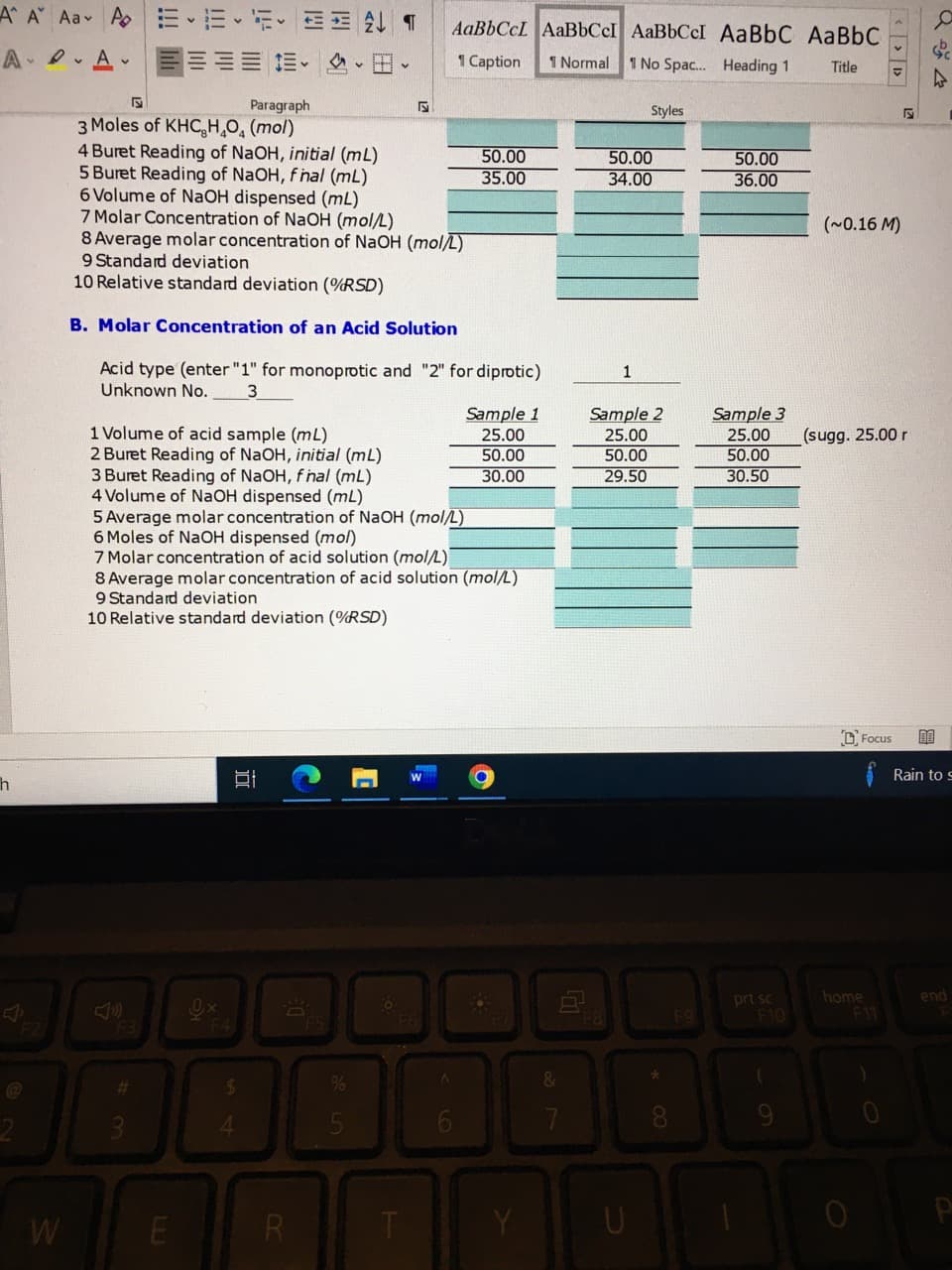
3 Moles of KHC,H,O, (mol)
4 Buret Reading of NaOH, initial (mL)
5 Buret Reading of NaOH, f hal (mL)
6 Volume of NaOH dispensed (mL)
7 Molar Concentration of NaOH (mol/L)
8 Average molar concentration of NaOH (mol/L)
9 Standard deviation
10 Relative standard deviation (%RSD)
50.00
35.00
50.00
34.00
50.00
36.00
(~0.16 M)
B. Molar Concentration of an Acid Solution
Acid type (enter "1" for monoprotic and "2" for diprotic)
Unknown No.
1
Sample 3
25.00
Sample 2
25.00
50.00
29.50
Sample 1
1 Volume of acid sample (mL)
2 Buret Reading of NaOH, initial (mL)
3 Buret Reading of NaOH, f hal (mL)
4 Volume of NAOH dispensed (mL)
5 Average molar concentration of NaOH (mol/L)
6 Moles of NaOH dispensed (mol)
7 Molar concentration of acid solution (mol/L)
8 Average molar concentration of acid solution (mol/L)
9 Standard deviation
10 Relative standard deviation (%RSD)
25.00
(sugg. 25.00 r
50.00
30.00
50.00
30.50
D Focus
Rain to s
home
F11
end
prt sc
F10
F3
5
E
R
00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

