b. Identify the unknown compound from the 3 possible choices in the table below. The physical properties of all three possible compounds are shared to help you identify the compound. Then in your own words explain how you identified the compound, what information did you use and how does it support your choice. Answers should be formatted as 1-2 complete sentences.
b. Identify the unknown compound from the 3 possible choices in the table below. The physical properties of all three possible compounds are shared to help you identify the compound. Then in your own words explain how you identified the compound, what information did you use and how does it support your choice. Answers should be formatted as 1-2 complete sentences.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 140AP: he following demonstration takes place in a two-step process: rst, solid calcium carbide...
Related questions
Question
100%
Using the table in the 2nd pic answer 5 b only

Transcribed Image Text:5. A cooling curve for an unknown compound is shown below. Use the information in the cooling curve to
identify the unknown compound from three possibilities listed in the table below.
a. Label the cooling curve with the states of matter (solid, liquid, gas) and the appropriate phase
changes.
b. Identify the unknown compound from the 3 possible choices in the table below. The physical
properties of all three possible compounds are shared to help you identify the compound. Then in
your own words explain how you identified the compound, what information did you use and
how does it support your choice. Answers should be formatted as 1-2 complete sentences.
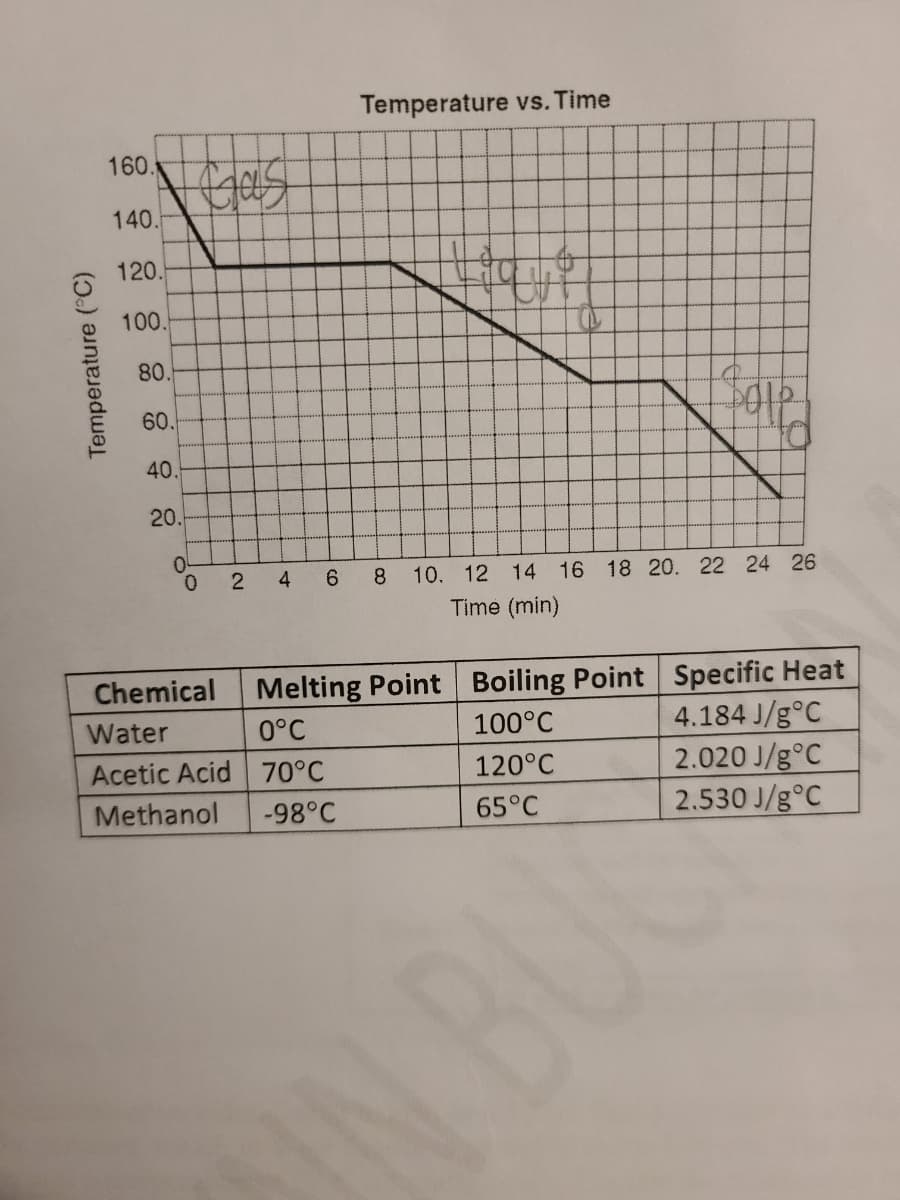
Transcribed Image Text:Temperature vs. Time
160.
140.
120.
100.
80.
60.
40.
20.
0.
6 8 10. 12 14 16 18 20. 22 24 26
Time (min)
Chemical
Melting Point Boiling Point Specific Heat
Water
0°C
4.184 J/g°C
2.020 J/g°C
2.530 J/g°C
100°C
Acetic Acid 70°C
120°C
Methanol
-98°C
65°C
NBUC
Temperature (°C)
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax