B. If 25.0 kJ of heat is lost by 0.500 mol of substance A initially at 110 °C, what is the final temperature of substance A? For substance A: molar heat of vaporization = 43.4 kJ/mol molar heat of fusion = 4.9 kJ/mol molar heat capacity of the liquid = 112.4 J/mol-K molar heat capacity of the gas = 37.12 J/mol-K Select one: Oa. 52 °C Ob. 30 °C Oc. 78 °C Od. 83 °C
B. If 25.0 kJ of heat is lost by 0.500 mol of substance A initially at 110 °C, what is the final temperature of substance A? For substance A: molar heat of vaporization = 43.4 kJ/mol molar heat of fusion = 4.9 kJ/mol molar heat capacity of the liquid = 112.4 J/mol-K molar heat capacity of the gas = 37.12 J/mol-K Select one: Oa. 52 °C Ob. 30 °C Oc. 78 °C Od. 83 °C
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 37QRT
Related questions
Question

Transcribed Image Text:B. If 25.0 kJ of heat is lost by 0.500 mol of substance A initially at 110 °C, what is the final temperature of
substance A?
For substance A:
molar heat of vaporization = 43.4 kJ/mol
molar heat of fusion = 4.9 kJ/mol
molar heat capacity of the liquid = 112.4 J/mol:K
molar heat capacity of the gas = 37.12 J/mol-K
Select one:
Oa. 52 °C
Ob. 30 °C
Oc. 78 °C
Od. 83 °C
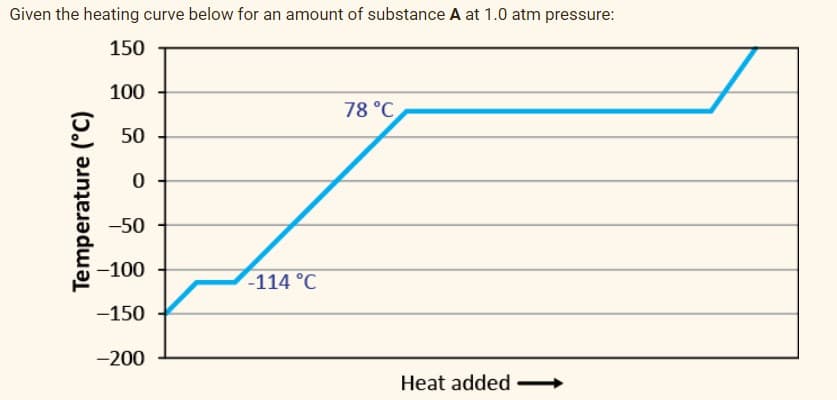
Transcribed Image Text:Given the heating curve below for an amount of substance A at 1.0 atm pressure:
150
100
78 °C
50
-50
-100
-114 °C
-150
-200
Heat added
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning
