Baking soda is a pure substance, sodium bicarbonate. Vinegar is a mixture of acetic acid dissolved in water, and it is only the acetic acid that reacts. Acetic acid and sodium bicarbonate tan react together according to the chemical equation below: HC2H302 NaHCOP NaC2H302 + H20 + Co2 acetic acid + sodium bicarbonate sodium acetate + water + carbon dioxide Samuel wants to help his brother make a science fair volcano. 1. For his first experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes the two powders together, but there is no reaction. b) Considering the ståte of matter of the particles, give a possible explanation for why there is no reaction.
Baking soda is a pure substance, sodium bicarbonate. Vinegar is a mixture of acetic acid dissolved in water, and it is only the acetic acid that reacts. Acetic acid and sodium bicarbonate tan react together according to the chemical equation below: HC2H302 NaHCOP NaC2H302 + H20 + Co2 acetic acid + sodium bicarbonate sodium acetate + water + carbon dioxide Samuel wants to help his brother make a science fair volcano. 1. For his first experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes the two powders together, but there is no reaction. b) Considering the ståte of matter of the particles, give a possible explanation for why there is no reaction.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 97E
Related questions
Question
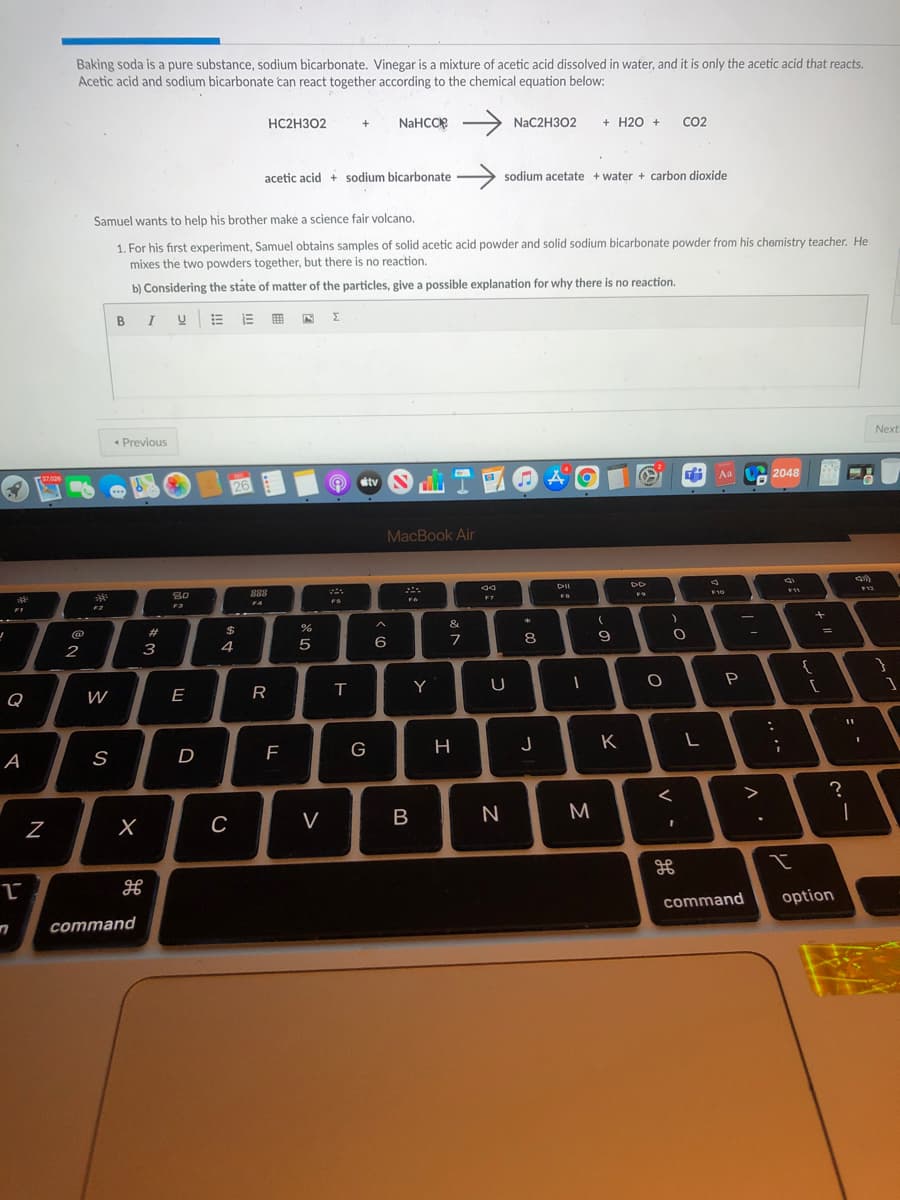
Transcribed Image Text:Baking soda is a pure substance, sodium bicarbonate. Vinegar is a mixture of acetic acid dissolved in water, and it is only the acetic acid that reacts.
Acetic acid and sodium bicarbonate can react together according to the chemical equation below:
HC2H302
NaHCOe
NAC2H302
+ H20 +
CO2
acetic acid + sodium bicarbonate
sodium acetate + water + carbon dioxide
Samuel wants to help his brother make a science fair volcano.
1. For his first experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He
mixes the two powders together, but there is no reaction.
b) Considering the state of matter of the particles, give a possible explanation for why there is no reaction.
BIUEE O
Σ
Next
* Previous
i Aa
2048
37.006
stv
MacBook Air
888
F11
F10
80
FS
F4
F3
F2
F1
+
&
@
2#
$
6
7
8
2
3
4
Y
Q
E
R
G
J
K
A
S
D
F
>
V
B
M
с
command
option
command
.. ·-
>
N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning