Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 64A
Related questions
Question
![Application A typical application of linear systems to chemistry is balancing a chemical
equation. The rationale behind this is the Law of conservation of mass which states the
following:
"mass is neither created nor destroyed in any chemical reaction. Therefore balancing of
equations requires the same number of atoms on both sides of a chemical reaction. The
mass of all the reactants (the substances going into a reaction) must equal the mass of the
products (the substances produced by the reaction)."
As an example consider the following chemical equation
CH + O → CO, + H,O.
Balancing this chemical reaction means finding values of x, y, z and w so that the number of
atoms of each element is the same on both sides of the equation:
xC₂H6+ YO2 → ZCO2+ wH₂0.
This gives the following linear system:
(2x = z
6x = 2w
2y = 2z+w
The general solution of the above system is
X=
y =
Z=
WE
7w
2w
XAN 3
y
W
"C"
"H"
"0"
= W
[1/3]
7/6
2/3
choosing w = 6 gives
ہو"
y
N 3
W
27
4
6
Since we are looking for whole values of the variables x, y z, and w, choose x=2 and get y-7, z=
4 and w-6. The balanced equation is then:
2C2H 6+ 702 4CO2+ 6H₂O.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F510b87f1-1062-4180-bcf7-c90378c42705%2F912835cb-0332-46d3-8451-7ca4d78e0d18%2Fm1t8v7h_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Application A typical application of linear systems to chemistry is balancing a chemical
equation. The rationale behind this is the Law of conservation of mass which states the
following:
"mass is neither created nor destroyed in any chemical reaction. Therefore balancing of
equations requires the same number of atoms on both sides of a chemical reaction. The
mass of all the reactants (the substances going into a reaction) must equal the mass of the
products (the substances produced by the reaction)."
As an example consider the following chemical equation
CH + O → CO, + H,O.
Balancing this chemical reaction means finding values of x, y, z and w so that the number of
atoms of each element is the same on both sides of the equation:
xC₂H6+ YO2 → ZCO2+ wH₂0.
This gives the following linear system:
(2x = z
6x = 2w
2y = 2z+w
The general solution of the above system is
X=
y =
Z=
WE
7w
2w
XAN 3
y
W
"C"
"H"
"0"
= W
[1/3]
7/6
2/3
choosing w = 6 gives
ہو"
y
N 3
W
27
4
6
Since we are looking for whole values of the variables x, y z, and w, choose x=2 and get y-7, z=
4 and w-6. The balanced equation is then:
2C2H 6+ 702 4CO2+ 6H₂O.
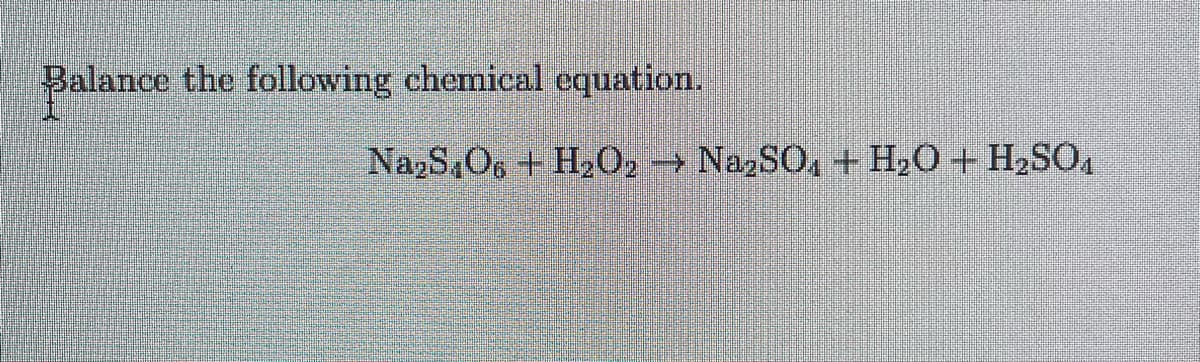
Transcribed Image Text:Balance the following chemical equation.
Na₂S O6 + H₂O2 → Na₂SO4 + H₂O + H₂SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning