Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C,H,(g) + O;(g) → co-(g) + H,O(g) (b) NH,NO;(s) –→ N‚O(g) + H,O(g) (c) C;H¿O(1) + O2(8) → CO2(g) + H;O(g) (d) N2(g) + H;(g) → NH;(g) (e) K¿O(s) + H,O(1) –→ KOH(aq)
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C,H,(g) + O;(g) → co-(g) + H,O(g) (b) NH,NO;(s) –→ N‚O(g) + H,O(g) (c) C;H¿O(1) + O2(8) → CO2(g) + H;O(g) (d) N2(g) + H;(g) → NH;(g) (e) K¿O(s) + H,O(1) –→ KOH(aq)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter29: Supercritical Fluid Chromatography And Extraction
Section: Chapter Questions
Problem 29.11QAP
Related questions
Question
Hello, can you please help with # 3.21
Thanks
Transcribed Image Text:Preview
File
Edit
View
Go
Tools
Window
Help
Sun Jun 6 9:47 PM
Theodore E. Brown, H. Eugene H LeMay, Bruce E. Bursten, Cather...
Page 153 of 1,246
Q Search
- Shared
WIICII (a) Ivig(- itacis VWICII CI2\X)2 (U val luiII Cal vollait ut-
Theodore E. Brown, H. Eu...
Benzaldehyde
(almond fragrance)
composes into barium oxide and carbon dioxide
when
gas
heated; (c) the hydrocarbon styrene, C3H3(1), is combusted in
air; (d) dimethylether, CH;OCH3(g), is combusted in air.
(а) Н—С
С-С—н
Exercises
Waling Cete
w em l
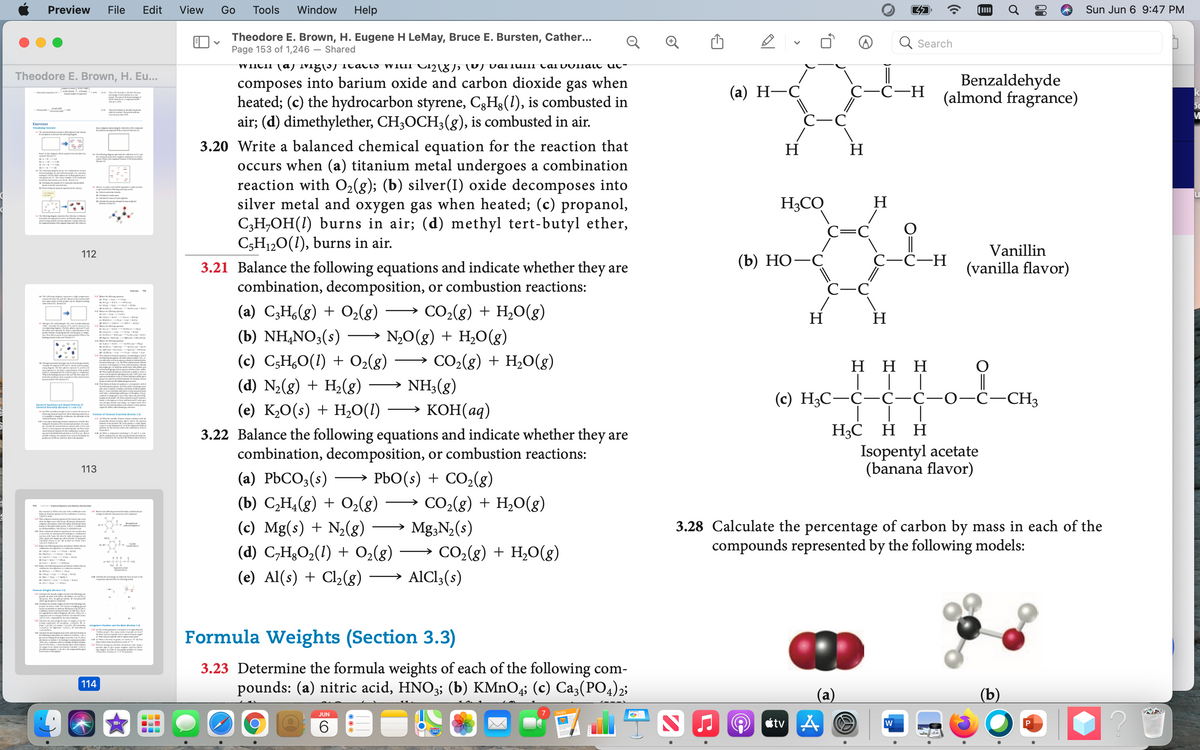
3.20 Write a balanced chemical equation for the reaction that
occurs when (a) titanium metal undergoes a combination
reaction with O2(g); (b) silver(I) oxide decomposes into
silver metal and oxygen gas when heated; (c) propanol,
C3H,OH(1) burns in air; (d) methyl tert-butyl ether,
C5H120(1), burns in air.
H
H
t
H3CO
H
eme m fe
C=C
Vanillin
112
(b) НО—С
C-C-H
3.21 Balance the following equations and indicate whether they are
combination, decomposition, or combustion reactions:
(vanilla flavor)
C-C
(a) C3H,(g) + O2(g)
CO2(g) + H2O(g)
H
H
r en
(b) NH,NO3(s)
→ N,O(g) + H2O(g)
NE
(c) C5H,O(1) + O2(g) → CO2(g) + H,O(g)
→ NH3(g)
KOH(aq)
ннн
(d) N2(g) + H2(g)
(c) H3C-C-Ç-C-0-C-CH3
(e) K½O(s) + H,0(1)
Cial tiontple am
Cheial Biarty Se i t3
e ei
Pu ef Chumi y Se
3.22 Balance the following equations and indicate whether they are
combination, decomposition, or combustion reactions:
H3C H H
Isopentyl acetate
(banana flavor)
113
(a) РЬСО,(:)
→ PbO(s) + CO2(g)
CO,(g) + H20(g)
→ Mg,N2(s)
→ CO2(g) + H2O(g)
→ AICI;(s)
(b) C,H4(g) + O2(g)
D a
E kal e de
t me
(c) Mg(s) + N½(g)
(d) C,H3O2(1) + O2(g)
3.28 Calculate the percentage of carbon by mass in each of the
compounds represented by the following models:
(e) Al(s) + Cl2(g) -
G e ay
Twag
AM al
g's w an M 14
Formula Weights (Section 3.3)
e ed
wW ta
3.23 Determine the formula weights of each of the following com-
pounds: (a) nitric acid, HNO3; (b) KMnO4; (c) Ca3(PO4)2;
114
(b)
JUN
étv A
6.
w
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning