Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter15: Solutions Of Acids And Bases
Section: Chapter Questions
Problem 15.130QE: A solution is made by dissolving 15.0 g sodium hydroxide in approximately 450 mL water. The solution...
Related questions
Question
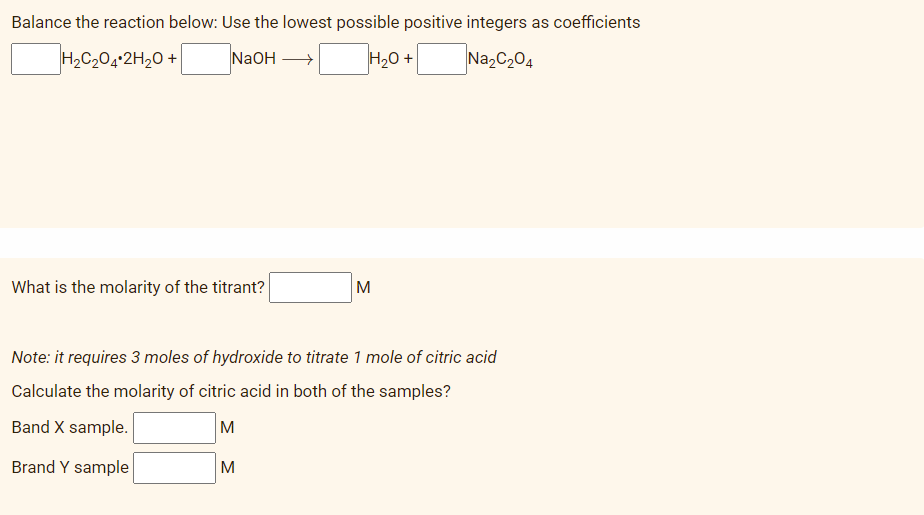
Transcribed Image Text:Balance the reaction below: Use the lowest possible positive integers as coefficients
H2C204-2H20 +
NaOH
H20 +
Na2C204
What is the molarity of the titrant?
M
Note: it requires 3 moles of hydroxide to titrate 1 mole of citric acid
Calculate the molarity of citric acid in both of the samples?
Band X sample.
M
Brand Y sample
M
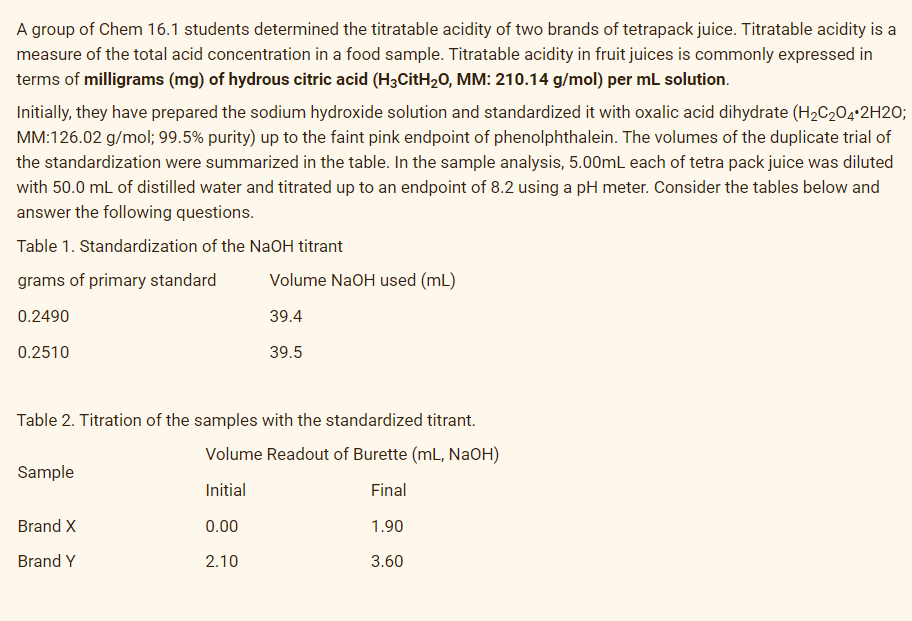
Transcribed Image Text:A group of Chem 16.1 students determined the titratable acidity of two brands of tetrapack juice. Titratable acidity is a
measure of the total acid concentration in a food sample. Titratable acidity in fruit juices is commonly expressed in
terms of milligrams (mg) of hydrous citric acid (H3CitH20, MM: 210.14 g/mol) per ml solution.
Initially, they have prepared the sodium hydroxide solution and standardized it with oxalic acid dihydrate (H2C204•2H2O;
MM:126.02 g/mol; 99.5% purity) up to the faint pink endpoint of phenolphthalein. The volumes of the duplicate trial of
the standardization were summarized in the table. In the sample analysis, 5.00mL each of tetra pack juice was diluted
with 50.0 mL of distilled water and titrated up to an endpoint of 8.2 using a pH meter. Consider the tables below and
answer the following questions.
Table 1. Standardization of the NaOH titrant
grams of primary standard
Volume NaOH used (mL)
0.2490
39.4
0.2510
39.5
Table 2. Titration of the samples with the standardized titrant.
Volume Readout of Burette (mL, NaOH)
Sample
Initial
Final
Brand X
0.00
1.90
Brand Y
2.10
3.60
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning