Balance the reaction between H3AsO4 and Zn to form HAsO2 and Zn2+ in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown. H3AsO4 + Zn HAsO2 + Zn2+ Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.) How many electrons are transferred in this reaction?
Balance the reaction between H3AsO4 and Zn to form HAsO2 and Zn2+ in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown. H3AsO4 + Zn HAsO2 + Zn2+ Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.) How many electrons are transferred in this reaction?
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 21Q: When magnesium metal is added to a beaker of HCl(aq), a gas is produced. Knowing that magnesium is...
Related questions
Question
100%
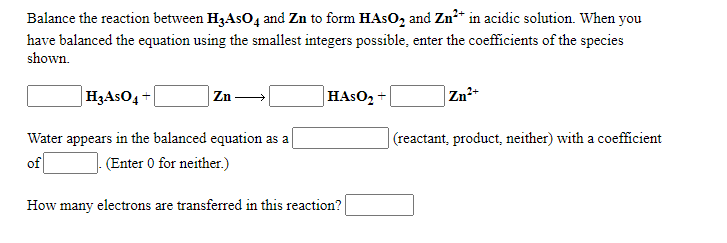
Balance the reaction between H3AsO4 and Zn to form HAsO2 and Zn2+ in acidic solution. When you have balanced the equation using the smallest integers possible, enter the coefficients of the species shown.
H3AsO4 + Zn HAsO2 + Zn2+
Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)
How many electrons are transferred in this reaction?
Transcribed Image Text:Balance the reaction between H3ASO4 and Zn to form HAS02 and Zn?* in acidic solution. When you
have balanced the equation using the smallest integers possible, enter the coefficients of the species
shown.
|H3ASO4
Zn-
HASO2
Zn+
Water appears in the balanced equation as a
(reactant, product, neither) with a coefficient
of
(Enter 0 for neither.)
How many electrons are transferred in this reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
