Q: What is the difference between General Test and Confirmatory test
A: Chemistry can be defined as the branch of science that deals with the composition, and structure of…
Q: D) NO
A: From the MOT configuration, the molecule will have 14 electrons So, NO+ has electrons is Number…
Q: O RBCI
A: Moles = mass / molar mass Molarity =Moles/Volume (L) As we know, Higher the molar mass of solute,…
Q: Hz S64. NH_ Na BH jCN
A:
Q: a) b) 0!
A: According to the question, we need to name the given organic compound.
Q: -C N:
A: There are two question, in hybridization asked in each.
Q: H. он D.
A: Less than 10 of pKa value means , the molecule has an acidic hydrogen. Less pKa value means more…
Q: Al + HCI > AICI3 + H2
A: The coefficient for each reactants and products has been given below
Q: ¹⁷org chem question answer with explanation
A: All these given reaction are of type : electrophilic aromatic substitution reaction. The details…
Q: HN. DMSO (solvent) MeO2S
A: It is an example of ipso substitution reaction.
Q: 1. PrMgBr CI 2. aq H* Meo
A: Here we are required to find the reagent as well as the mechanism for the given reaction to form the…
Q: SNL U ה C - d-1fC SNI
A:
Q: Confirmatory Test NaOH(aq) Test/reagent used: Chemical(s) responsible for a positive test:…
A:
Q: ille PPM
A: chemical shift values are written from spectra
Q: and
A: Answer-c Compounds those have same molecular formula but different structural formulas are called as…
Q: 44 delete TFntaime d. е.
A: In this question we have to tell the name of the given molecule.
Q: SOCI, 12.
A: Reaction of an alcohol with SOCl2 proceeds via SNi mechanism.
Q: c) NaOMe Ph МеОн OTs
A: For E2 elimination the two departing group H & -OTs should be anti to each other. At first, we…
Q: sequence: A A B B cc D D EE
A: The product of the following sequence is:
Q: (2 pts. each) 1. Given the e equation: PCl:(g) =
A: Given, Equilibrium reaction: PCl5(g) ---> PCl3(g) + Cl2(g) We need to determine the shift of…
Q: ne AH fc
A:
Q: *(d) P. HPO + PH3
A: Balance Redox reaction in basic medium : P4 -----> HPO32- + PH3
Q: Question 4 Replication in an analysis is required to O Get reliable data O Minimize error O Exhibit…
A: Two question based on tools in analytical chemistry that is to be accomplished.
Q: Do (b) (i)
A:
Q: Cd(s) + MgBr2(aq) →
A: This is single displacement reaction . Reaction in which one atom replace by another atom
Q: b) d) f) Et H. H "Ме Ме
A: The relationships are given below:
Q: ||| Br NaOCH3 A || IV
A:
Q: CHy'
A: The general skeleton of IUPAC nomenclature: Prefix + parent carbon chain/ring + suffix. Parent…
Q: lo.DL contamer at loo.0°C कनेवाळड कड व् ए०, ०.४००+m H>, १d 0.७५ वम्न् एमडु Oम- The ननान्wwng…
A: Equilibrium constant Kp is equal to partial pressure of products divided by partial pressure of…
Q: AHixn
A:
Q: AG°rxn II
A:
Q: Give the IUPAC name for each compound.
A: Since you have posted question with multiple sub-parts, we are entitled to answer the first 3 only.…
Q: °C
A:
Q: B1
A: To write the IUPAC name for the following:-
Q: Can someone please answer E-H! thank you
A:
Q: ANSWER ONLY 4 5 AND 6 SUBJECT ANALYTIC CHEMISTRY
A: Qualitative analysis is a part of analytical chemistry that involves determining the elements or…
Q: CHM220 Page 3 он m. CH3 n. HC
A:
Q: NACN DMSO Br
A:
Q: No 13
A: In the redox reaction, the oxidation and reduction reactions occur simultaneously. The oxidation…
Q: Convert 78mminto dm. Cuse 3 coersion facto
A: Given -78 mm Used formula- 1dm=100mm
Q: Please Answer quickly
A: Given that : % of n-octane = 25% by mass = 25 g So, % of benzene = (100-25)% = 75% by mass = 75 g…
Q: nthetic analysis a NH
A:
Q: icrowave radiation Itraviolet light nfrared radiation yellow light
A: Visible spectrum consist of seven colors radiations (VIBGYOR) So, Microwave radiation Ultraviolet…
Q: cerrcet Auduct socą EtgN noreachon
A: Reaction of ether with thionyl chloride. Correct option is D. No reaction will occur.
Q: d) Draw t e) Compa
A: Biomolecules are molecules found in living organisms. They are mainly compromised of carbon,…
Q: ct-
A: The total amount of metter in the universe remains constant. This is a first law of thermodynamics…
Q: जोपड व तायफरवलाड्ल निम कक् षजरकी डिळल)०्दूण्णा ve a OH
A:
Q: a. + SOCI, -> soclz O-H b. + エZー
A:
Q: Choose the best answer among the choices under each question/ statement. Thank you
A: There is a high amount of risk in laboratory that should be prevented by significant care and should…
Q: Select the best answer. .CI HO, EtN "R"abbreviation for answers a) b.) d)
A:
Balanced Chem Eq
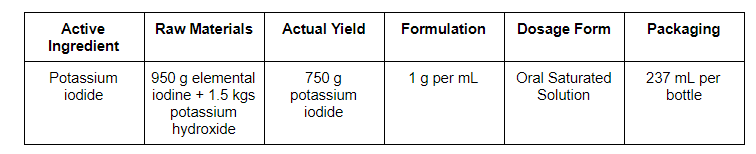
Step by step
Solved in 2 steps with 1 images

- Was the gradual color change observed when the sodium thiosulfate (Na2S2O3) crystal was added to the aqueous solution of KI/12 in Station Cevidence of a chemical or physical change?Pyrolusite (MnO2) is dissolved in hydrochloric acid:MnO2 + HCl → MnCl2 + H2O + Cl2The chloride was passed into potassium iodide solution where it liberated iodine:Cl2 + KI → KCl + I2The iodine liberated was estimated by adding sodium thiosulfate, the reaction beingI2 + Na2S2O3 → NaI + Na2S4O6If 5.6 g of crystallized sodium thiosulfate, Na2S2O3 5H2O ,were used up, how many grams of manganese were present?MW (g/mole): I =126.9; Mn =54.94; Cl = 35.45; K = 39.09; Na = 23; S = 32Balance the following chemical equation using the smallest possible wholenumber coefficients: P4 + Cl2 → PCl3A. 2 P4 + 6 Cl2 → 8 PCl3B. P4 + Cl2 → 4 PCl3C. P4 + 6 Cl2 → 4 PCl3D. P4 + 16 Cl2 → 4 PCl3
- In the synthesis of benzoic acid, 3.5 mL of toluene were used and mixed with potassium permanganate solution. In making the potassium permanganate solution, 7 grams of the powder were dissolved in 150 mL of water. The resulting crystals were purified and the yield 1.53 grams. Identify the limiting reagent and compute for the number of moles that it consumed. What is the theoretical yield? What is the percentage yield? MW toluene = 94.14, density=0.87 g/mL , MW KMnO4 = 158, density = 2.7 g/mL , MW Benzoic acid = 122, density = 1.27 g/mLVanadic ion, V3+, forms green salts and is a good reducingagent, being itself changed in neutral solutions to thenearly colorless ion V(OH)4+. Suppose that 15.0 mL of a0.200-M solution of vanadic sulfate, V2(SO4)3, was neededto reduce completely a 0.540-g sample of an unknownsubstance X. If each molecule of X accepted just oneelectron, what is the molecular weight of X? Suppose thateach molecule of X accepted three electrons; what wouldbe the molecular weight of X then?A technician is setting up a laboratory to standardise ~0.0200 mol dm–3 KMnO4. What mass of Mohr’s salt [FeSO4(NH4)2SO4·6H2O] would the technician be required to weigh out to produce a 5.00 L solution of Mohr’s salt such that 25.0 cm3 aliquots of the salt would require a titre of 20.0 cm3 to standardise the KMnO4 solution?
- 1. Write a balanced equation for the reaction between sodium carbonate (Na2CO3) andhydrochloric acid (HCl).2. Calculate the theoretical yield (in grams) of the CO2 that should be produced from the massof Na2CO3 used. Use dimensional analysis.3. Use the following equation to calculate the percent yield of the CO2.experimental(actual) %yield x 100theoretical(calculated)=4. Calculate the theoretical yield of NaCl from the amount of Na2CO3 used. Use dimensionalanalysis.5. Use the following equation to calculate the percent yield of the NaCl in your experiment.experimental(actual) %yield x 100theoretical(calculated)What constitutes a 'reference material', and why does its utilization play a criticalrole in the chemical analysis of food products? Provide examples.Which are characteristics of a primary standard? has an anhydrous form as a well as hydrated form low cost stability in the atmosphere high purity low molar mass high molar mass low solubility
- The mercury in a 0.8142-g sample was precipitated with an excess of paraperiodic acid, H5IO6: 5 Hg 2+ + 2 H5IO6 ---> Hg5(IO6)2 (s) + 10 H + The precipitate (MW = 1448.8 g/mol) was filtered, washed free of precipitating agent, dried, and weighed, and 0.4114 g was recovered. Calculate the a) % Hg (200.6 g/mol) b) % Hg2Cl2 (472.1 g/mol)Souring of wine occurs when ethanol is converted to acetic acid by oxygen: C2H5OH(l) +O2(g)--->CH3COOH(g)+H2O(l). A 1.00 L bottle of wine labeled as 7.00% (%v/v) ethanol, is found to have a defective seal. Analysis of 1.00 mL showed that there were 0.0274 g acetic acid in that 1.00 mL. The density of ethanol is 0.816 g/mL. (a) What mass of oxygen must leaked into the bottle in pounds(lb) (b) What is the percent yield for the conversion of ethanol to acetic acid, if oxygen is in excess2.Acrude compound with a mass of 0.62667 g was recrystalised to give 0.1352 g of crystals . Calculate the percentage recovery .Suggest possible reasons for the low percentage recovery

