Based on the ideal solubility equation, what solute characteristics indicate low solubility at normal temperature? A High melting point and large enthalpy B Low melting point and large enthalpy © High melting point and small enthalpy D Low melting point and small enthalpy 1. Which of the following refers to a composition in which its components cannot be separated by distillation? (A) Ideal B Eutectic Azeotropic D Congruent 2. The mixture of C and D deviates negatively to Raoult's law. Which interaction predominates? A) C-D B C-C only D-D only D) Both C-C and D-D C and D do not mix 3. In which phase boundary is the equation, InpERT, applicable? A Solid-liquid boundary B) Solid-vapor boundary © Liquid-vapor boundary
Based on the ideal solubility equation, what solute characteristics indicate low solubility at normal temperature? A High melting point and large enthalpy B Low melting point and large enthalpy © High melting point and small enthalpy D Low melting point and small enthalpy 1. Which of the following refers to a composition in which its components cannot be separated by distillation? (A) Ideal B Eutectic Azeotropic D Congruent 2. The mixture of C and D deviates negatively to Raoult's law. Which interaction predominates? A) C-D B C-C only D-D only D) Both C-C and D-D C and D do not mix 3. In which phase boundary is the equation, InpERT, applicable? A Solid-liquid boundary B) Solid-vapor boundary © Liquid-vapor boundary
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.65E
Related questions
Question

Transcribed Image Text:Based on the ideal solubility equation, what solute characteristics indicate low solubility at normal temperature?
A High melting point and large enthalpy
B Low melting point and large enthalpy
(c) High melting point and small enthalpy
D Low melting point and small enthalpy
1.
Which of the following refers to a composition in which its components cannot be separated by distillation?
(A Ideal
B) Eutectic
c) Azeotropic
D Congruent
2.
The mixture of C and D deviates negatively to Raoult's law. Which interaction predominates?
A) C-D
в) с-С only
c) D-D only
D) Both C-C and D-D
E) C and D do not mix
3.
In which phase boundary is the equation, InpR applicable?
(A) Solid-liquid boundary
B) Solid-vapor boundary
(c) Liquid-vapor boundary
4.
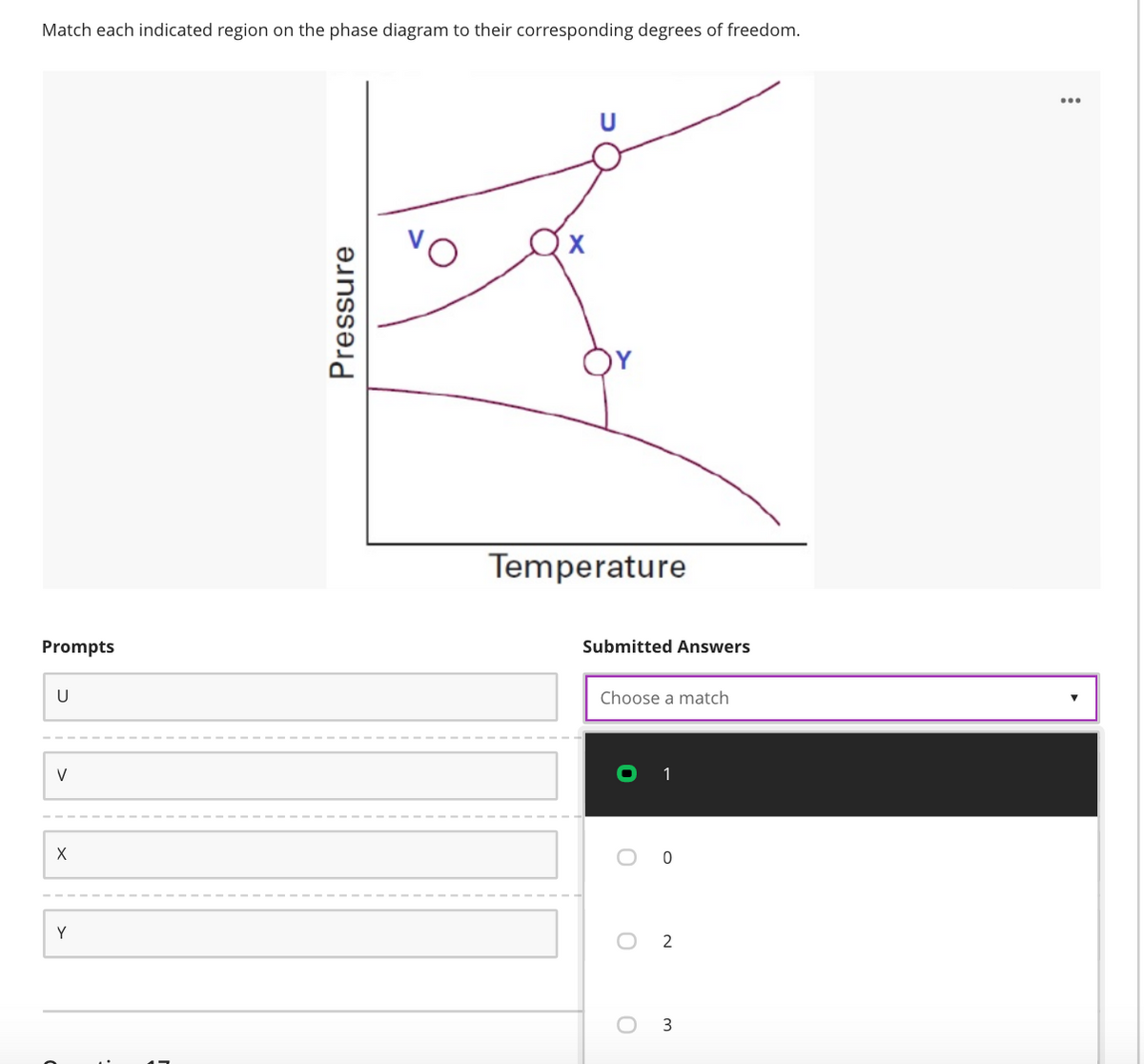
Transcribed Image Text:Match each indicated region on the phase diagram to their corresponding degrees of freedom.
Temperature
Prompts
Submitted Answers
U
Choose a match
V
O 1
Y
2
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning