Based on your ICE table and definition of Ka, set up the expression for Ka in order to determine the unknown. Do not combine or simplify terms. Ka = Ка = 1.8 x 10-5
Based on your ICE table and definition of Ka, set up the expression for Ka in order to determine the unknown. Do not combine or simplify terms. Ka = Ка = 1.8 x 10-5
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter14: Acid- Base Equilibria
Section: Chapter Questions
Problem 118CP: Malonic acid (HO2CCH2CO2H) is a diprotic acid. In the titration of malonic acid w ith NaOH,...
Related questions
Question
Using the blue bars as fillable selections in the ICE/RICE table, find the mass of NaCH3COO (I got the answer with Henderson Hasselbalch, but this problem requires the RICE table to be filled, and I'm unsure how to approach it).
![ul Verizon LTE
11:32
9 67%
Question 9 of 11
Submit
Determine the mass of solid NaCH3COO that must be
dissolved in an existing 500.0 mL solution of 0.200 M
CH;COOH to form a buffer with a pH equal to 5.00. The
value of Ka for CH;COOH is 1.8 x 10-5.
1
2
3
Based on your ICE table and definition of Ka, set up the
expression for Ka in order to determine the unknown.
Do not combine or simplify terms.
Ka =
= 1.8 x 10-5
5 RESET
[0]
[500.0]
[0.200]
[5.00]
[1.0 x 10-]
[1.0 x 10-5]
[1.8 x 10-]
[x + 5.00]
[x - 5.00]
[x + 1.0 x 10-]
[x - 1.0 x 10-°]
[x + 1.0 × 10-]
[x - 1.0 x 10-5]
[x + 1.8 x 10-5]
[x - 1.8 x 10-5]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fa5302e4a-fd12-4bcf-9790-b07cf8d45049%2F11637e93-08c4-48ed-9d20-ec2654a7e190%2Feaj1bin_processed.png&w=3840&q=75)
Transcribed Image Text:ul Verizon LTE
11:32
9 67%
Question 9 of 11
Submit
Determine the mass of solid NaCH3COO that must be
dissolved in an existing 500.0 mL solution of 0.200 M
CH;COOH to form a buffer with a pH equal to 5.00. The
value of Ka for CH;COOH is 1.8 x 10-5.
1
2
3
Based on your ICE table and definition of Ka, set up the
expression for Ka in order to determine the unknown.
Do not combine or simplify terms.
Ka =
= 1.8 x 10-5
5 RESET
[0]
[500.0]
[0.200]
[5.00]
[1.0 x 10-]
[1.0 x 10-5]
[1.8 x 10-]
[x + 5.00]
[x - 5.00]
[x + 1.0 x 10-]
[x - 1.0 x 10-°]
[x + 1.0 × 10-]
[x - 1.0 x 10-5]
[x + 1.8 x 10-5]
[x - 1.8 x 10-5]
Transcribed Image Text:ul Verizon LTE
11:32
9 67%
Question 9 of 11
Submit
Determine the mass of solid NaCH3COO that must be
dissolved in an existing 500.0 mL solution of 0.200 M
CH;COOH to form a buffer with a pH equal to 5.00. The
value of Ka for CH;COOH is 1.8 x 10-5.
1
2
3
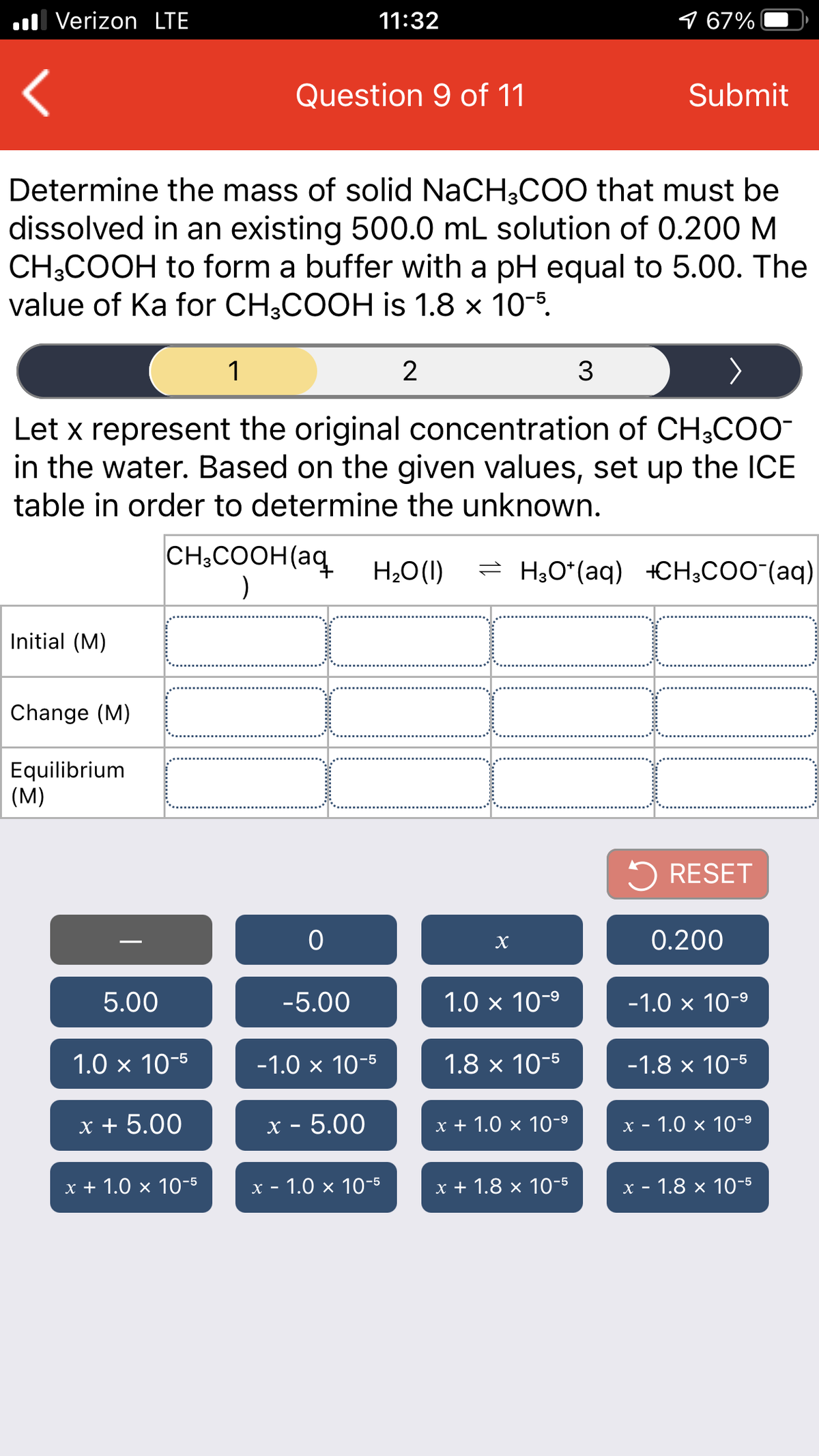
Let x represent the original concentration of CH3COO-
in the water. Based on the given values, set up the ICE
table in order to determine the unknown.
CH;COOH(aq
H20(1)
= H;O*(aq) +CH;COO-(aq)
Initial (M)
Change (M)
Equilibrium
(M)
5 RESET
0.200
5.00
-5.00
1.0 x 10-9
-1.0 x 10-9
1.0 x 10-5
-1.0 x 10-5
1.8 x 10-5
-1.8 x 10-5
x + 5.00
x - 5.00
х+1.0 x 10-°
х - 1.0 х 10-9
x + 1.0 × 10-5
х - 1.0 x 10-5
x + 1.8 × 10-5
х - 1.8 х 10-5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning