1.A student performed an experiment to find the number of water molecules associated with the CuSO, x H,O, and obtained the following data. Using the data given, find the number of water molecules associated with CuSO4 x H,O, and also find out the percentage of water in the hydrate.
1.A student performed an experiment to find the number of water molecules associated with the CuSO, x H,O, and obtained the following data. Using the data given, find the number of water molecules associated with CuSO4 x H,O, and also find out the percentage of water in the hydrate.
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.1QAP
Related questions
Question
100%
I don't know.. all of them..
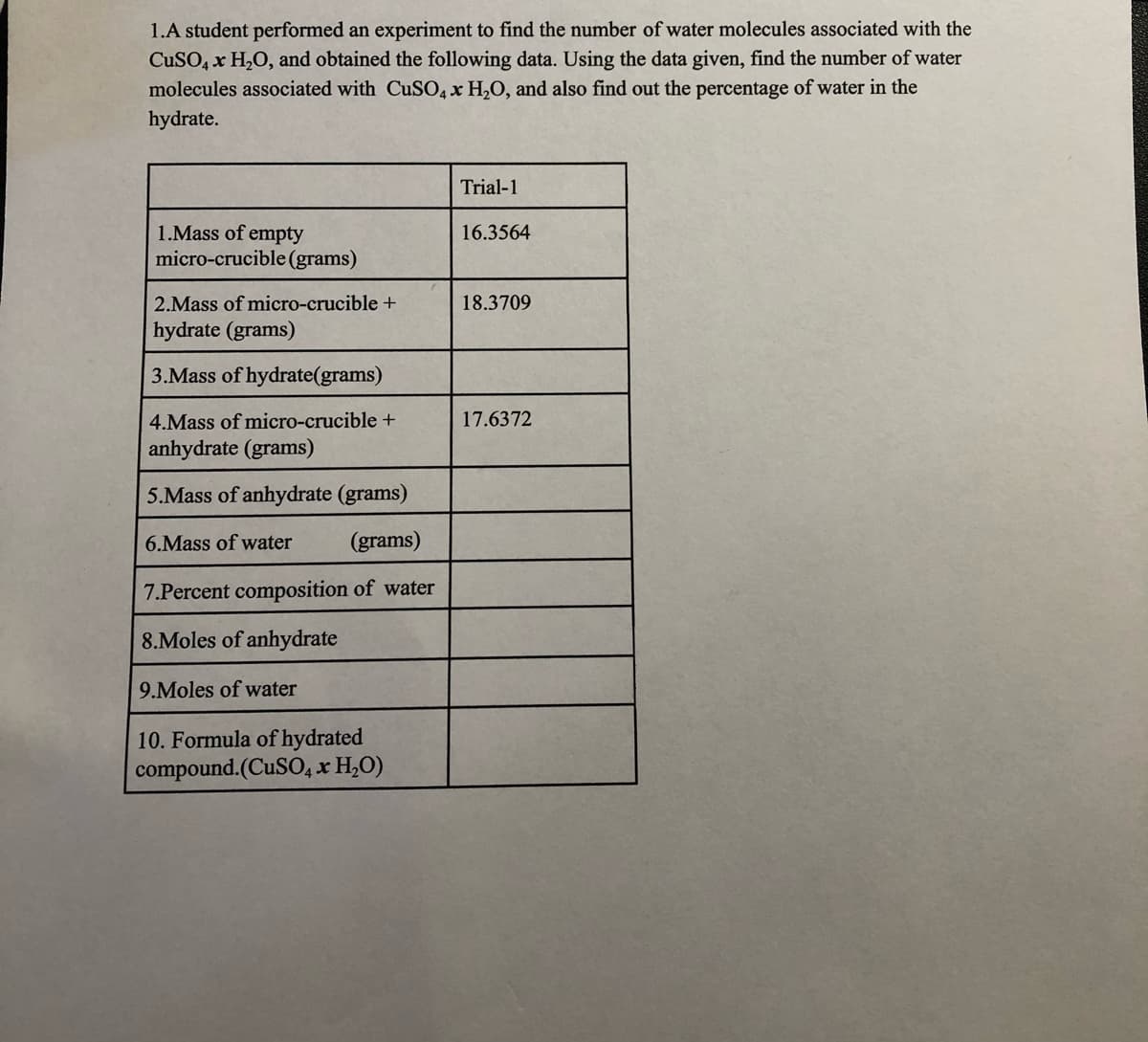
Transcribed Image Text:1.A student performed an experiment to find the number of water molecules associated with the
CuSO, x H2O, and obtained the following data. Using the data given, find the number of water
molecules associated with CuSO, x H2O, and also find out the percentage of water in the
hydrate.
Trial-1
1.Mass of empty
micro-crucible (grams)
16.3564
2.Mass of micro-crucible +
18.3709
hydrate (grams)
3.Mass of hydrate(grams)
4.Mass of micro-crucible +
17.6372
anhydrate (grams)
5.Mass of anhydrate (grams)
6.Mass of water
(grams)
7.Percent composition of water
8.Moles of anhydrate
9.Moles of water
10. Formula of hydrated
compound.(CUSO4 x H,O)
![All of them!
Calculations:
a. Mass of hydrate:=[(Mass of micro-crucible + hydrate) - (Mass of empty micro-crucible)]
b. Mass of anhydrate: =[(Mass of micro-crucible + anhydrate) - (Mass of empty micro-crucible)]
c. Mass of water: =[(Mass of hydrate) - (Mass of anhydrate)]
Mass of water
d. Percentage of water in the hydrate (%) =
-x100
Mass of hydrate
e. Moles of anhydrate :
Mass of anhydrate(g) x Molar mass of anhydrate (g)
1 mol
f. Moles of water :
1 mol
Molar mass of water(g)
Mass of water (g) x
Moles of water
g. Number of molecules of water(x) =
Moles of anhydrate
(report this answer in two significant figures)
Answer the following:
1. The formula for ibuprofen is C13H18O2. How many moles of ibuprofen are present in
250.0-mg tablet?
2. Given the following molecular formulas, determine the empirical formula of each compound:
N,O5, PCl, H,O2, C,H,Cl,.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F400ddd21-ac80-46c1-ac1b-5f37c5528f5e%2F4139159a-9257-4099-b9b5-d3b2f9adae3b%2Ftihsl8r_processed.jpeg&w=3840&q=75)
Transcribed Image Text:All of them!
Calculations:
a. Mass of hydrate:=[(Mass of micro-crucible + hydrate) - (Mass of empty micro-crucible)]
b. Mass of anhydrate: =[(Mass of micro-crucible + anhydrate) - (Mass of empty micro-crucible)]
c. Mass of water: =[(Mass of hydrate) - (Mass of anhydrate)]
Mass of water
d. Percentage of water in the hydrate (%) =
-x100
Mass of hydrate
e. Moles of anhydrate :
Mass of anhydrate(g) x Molar mass of anhydrate (g)
1 mol
f. Moles of water :
1 mol
Molar mass of water(g)
Mass of water (g) x
Moles of water
g. Number of molecules of water(x) =
Moles of anhydrate
(report this answer in two significant figures)
Answer the following:
1. The formula for ibuprofen is C13H18O2. How many moles of ibuprofen are present in
250.0-mg tablet?
2. Given the following molecular formulas, determine the empirical formula of each compound:
N,O5, PCl, H,O2, C,H,Cl,.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

