BASIC STOICHIOMETRY T01/S21 Based on the balanced equation, Molar Mass (g/mol) 3TIO2 + 4C + 6CI2 - 2C02 + 2C0 + 3TICIA TiO2 79.888 calculate the atoms of C required when 138 molecules of CO are formed. 12.011 Cl, 70.905 CO2 44.010 28.010 189.69 CO TiCl4 Avogadro's No. 6.022x1023 mol1 exact number, no tolerance Question Attempts: 0 of 1 used SAVE FOR LATER SUBMIT ANSWER
BASIC STOICHIOMETRY T01/S21 Based on the balanced equation, Molar Mass (g/mol) 3TIO2 + 4C + 6CI2 - 2C02 + 2C0 + 3TICIA TiO2 79.888 calculate the atoms of C required when 138 molecules of CO are formed. 12.011 Cl, 70.905 CO2 44.010 28.010 189.69 CO TiCl4 Avogadro's No. 6.022x1023 mol1 exact number, no tolerance Question Attempts: 0 of 1 used SAVE FOR LATER SUBMIT ANSWER
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 54QAP: Ammonia reacts with a limited amount of oxygen according to the equation...
Related questions
Question
Please provide detailed steps as I am using this to study, along with explanations as to what you did on each step
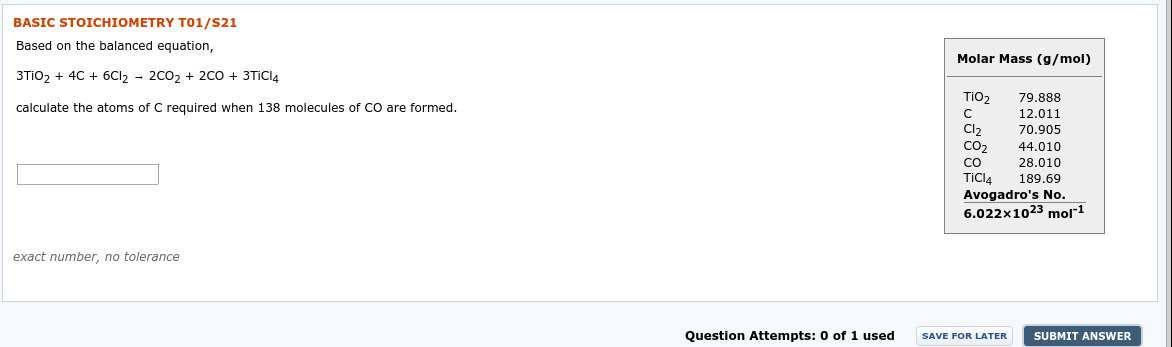
Transcribed Image Text:BASIC STOICHIOMETRY T01/S21
Based on the balanced equation,
Molar Mass (g/mol)
3TIO2 + 4C + 6CI2 - 2C02 + 2C0 + 3TICIA
TiO2
79.888
calculate the atoms of C required when 138 molecules of CO are formed.
12.011
Cl,
70.905
CO2
44.010
28.010
189.69
CO
TiCl4
Avogadro's No.
6.022x1023 mol1
exact number, no tolerance
Question Attempts: 0 of 1 used
SAVE FOR LATER
SUBMIT ANSWER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning