Be sure to answer all parts. Compounds A and B are isomers having molecular formula C3H12. Heating A with Cl, gives a single product of monohalogenation, whereas heating B under the same conditions forms three constitutional isomers. What are the structures of A and B?
Be sure to answer all parts. Compounds A and B are isomers having molecular formula C3H12. Heating A with Cl, gives a single product of monohalogenation, whereas heating B under the same conditions forms three constitutional isomers. What are the structures of A and B?
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter4: Acids And Bases
Section4.2: Brønsted-lowry Acids And Bases
Problem 4.4P: Write an equation to show the proton transfer between each alkene or cycloalkene and HCl. Where two...
Related questions
Question
Need help with Compound A.
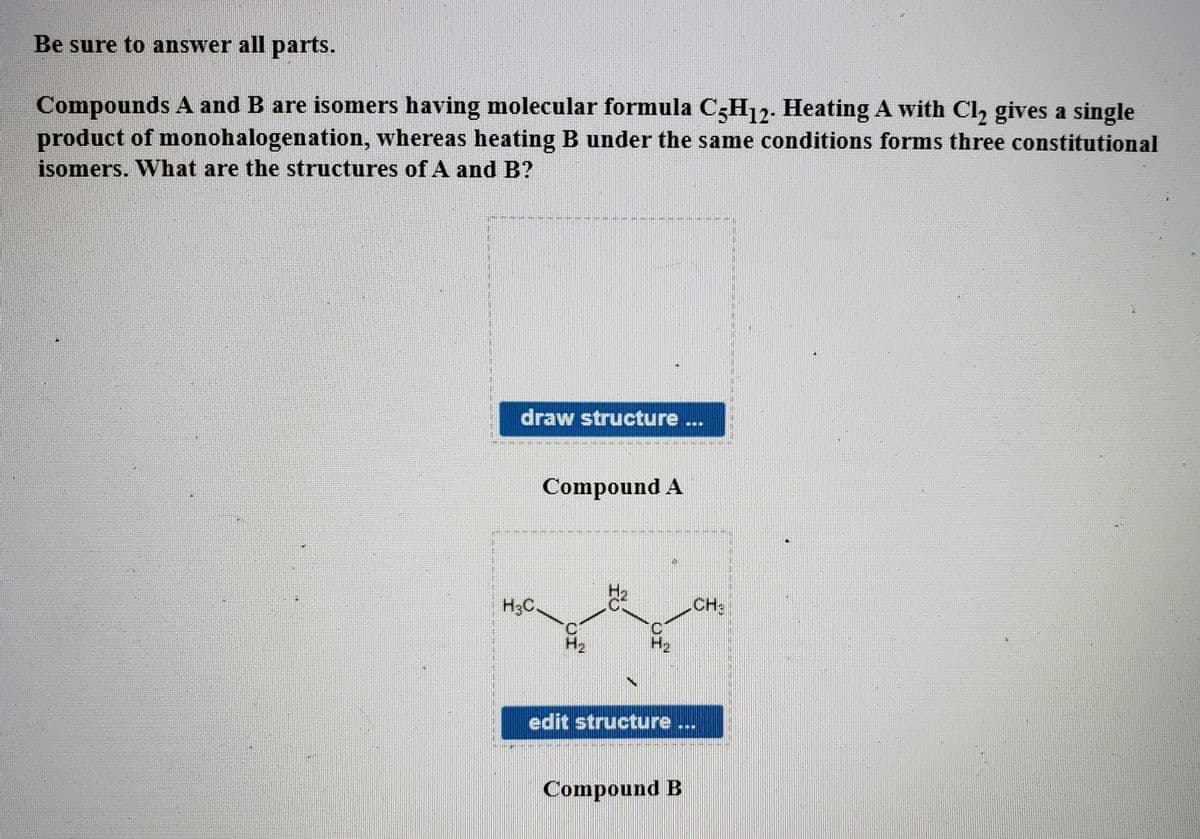
Transcribed Image Text:Be sure to answer all parts.
Compounds A and B are isomers having molecular formula C3H12. Heating A with Cl, gives a single
product of monohalogenation, whereas heating B under the same conditions forms three constitutional
isomers. What are the structures of A and B?
draw structure ...
Compound A
H3C.
CH:
H2
H2
edit structure ...
Compound B
OI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning