Be sure to answer all parts. Consider the reaction ДН -92.6 kJ/mol N2(g)+3H2(g) 2NH3(g) rxn If 2.0 moles of N2 react with 6.0 moles of H2 to form NH3, calculate the work done (in joules) against a pressure of 1.0 atm at 25°C. J What is AU for this reaction? Assume the reaction goes to completion. AU= kJ
Be sure to answer all parts. Consider the reaction ДН -92.6 kJ/mol N2(g)+3H2(g) 2NH3(g) rxn If 2.0 moles of N2 react with 6.0 moles of H2 to form NH3, calculate the work done (in joules) against a pressure of 1.0 atm at 25°C. J What is AU for this reaction? Assume the reaction goes to completion. AU= kJ
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.22E: What is the change in internal energy when a gas contracts from 377mL to 119mLundera pressure of...
Related questions
Question
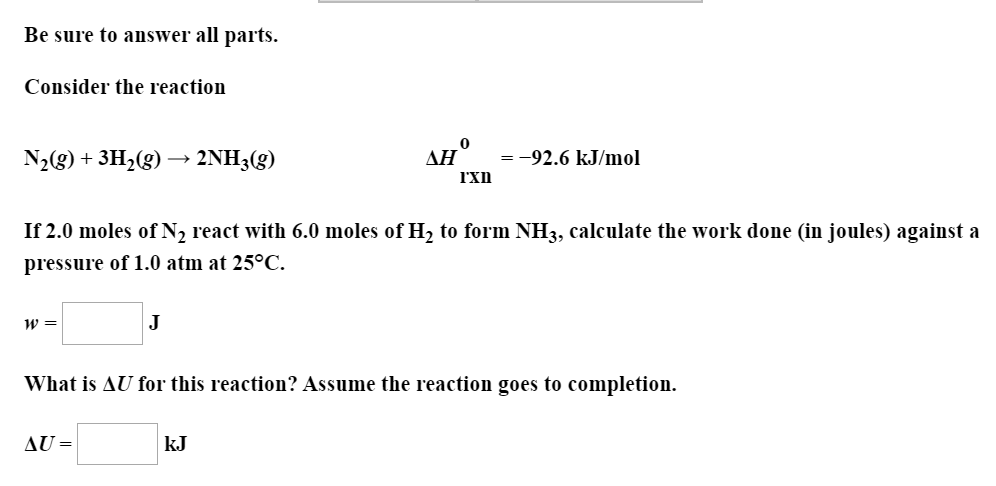
Transcribed Image Text:Be sure to answer all parts.
Consider the reaction
ДН
-92.6 kJ/mol
N2(g)+3H2(g) 2NH3(g)
rxn
If 2.0 moles of N2 react with 6.0 moles of H2 to form NH3, calculate the work done (in joules) against a
pressure of 1.0 atm at 25°C.
J
What is AU for this reaction? Assume the reaction goes to completion.
AU=
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning