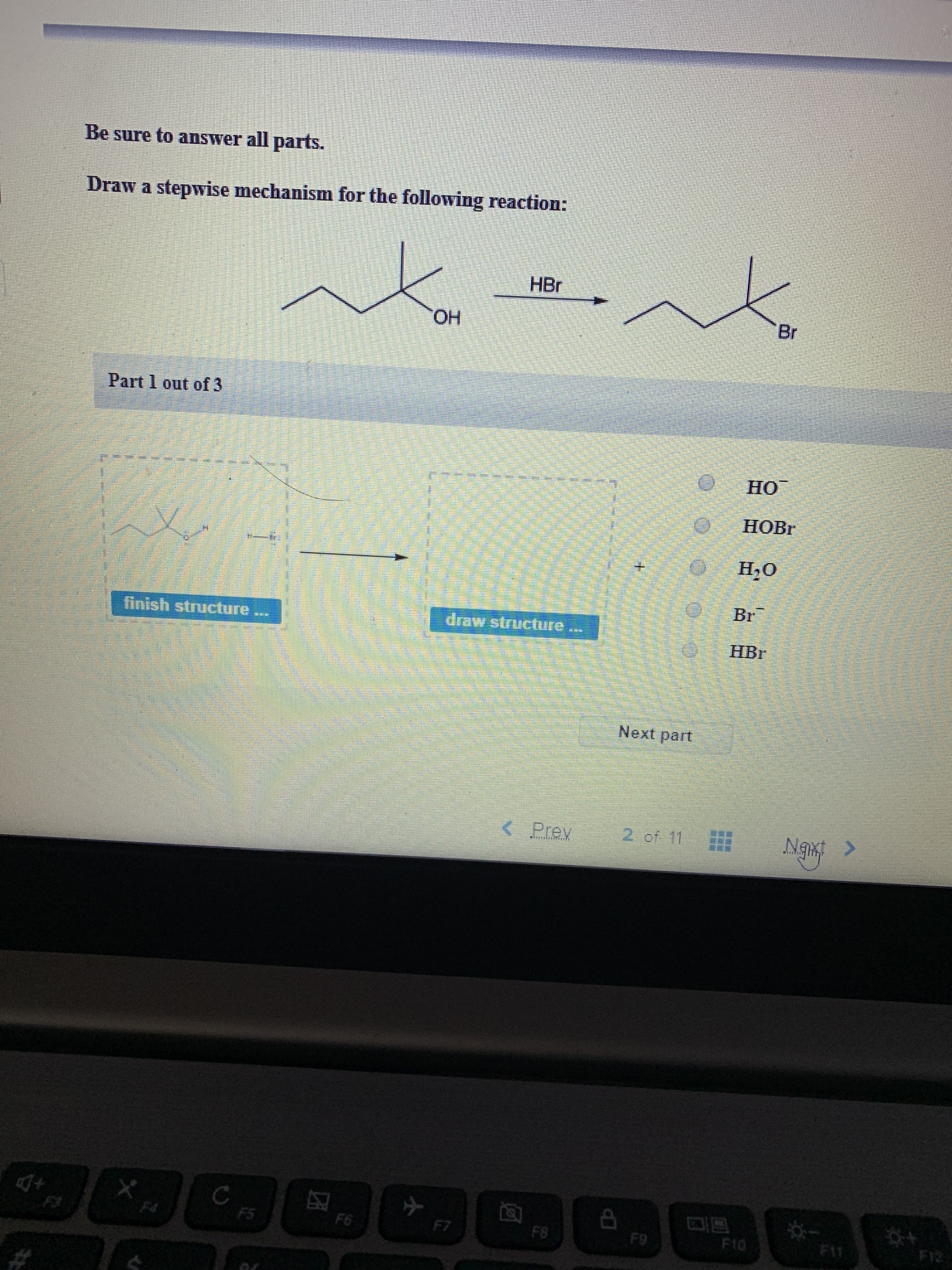
Be sure to answer all parts. Draw a stepwise mechanism for the following reaction: HBr Br ОН OH Part 1 out of 3 HO HOBR H2O Br draw structure finish structure HBr Next part of 11 Prev FI F11 F10 F8 F9 F6 F7 F5 F4
Q: stion 11 of 11 Draw the most stable form of the major mixed Claisen product formed in the reaction.…
A:
Q: Q#7 Draw a stepwise, detailed mechanism for the following reaction. Use curved arrows to show the…
A: The above Reaction is the Nucleophilic substitution reaction . In the Reaction the Alkyl Halide…
Q: Be sure to answer all parts. Predict the product and draw a stepwise mechanism for the following…
A: For the above conversion use unimolecular substitution reaction.
Q: Which of the following reagent best accomplish this transformation below? CH CH- CEC- A (1st) HCI,…
A:
Q: Which of the following are a major product of the reaction sequence shown below? (1) Br2, FeBry (2)…
A: Organic chemistry.
Q: OH Br CH3 CH3 CH CH3 aq. H2SO4. Br2 -or- CH3 CH3 H20 option #1 option #2
A:
Q: Draw the product and stepwise mechanism for the following reaction. 1. HCI, high temperature 2. HBr
A:
Q: -78 °C (CH3)3SICI Choose. + (CH2)½CCI ZnCl2
A: Please find the answer attached, here as handwritten note.j
Q: Be sure to answer all parts. Draw a stepwise mechanism for the following reaction: HBr HO. Br
A: Given reaction is, example of SN1 substitution reaction,
Q: Be sure to answer all parts. Predict the product and draw a stepwise mechanism for the following…
A: The given reaction will go through SN1 mechanism
Q: Be sure to answer all parts. Predict the product of the following reaction and then draw a stepwise…
A: Here, in the given reaction, Bromide ion substitutes the -OH group and leads to formation of Alkyl…
Q: Be sure to answer all parts. Draw a stepwise mechanism for the following reaction: HBr HO. Br Part…
A: Given reaction is the reaction of alcohol with strong acid to form alkyl halide. In this reaction,…
Q: Draw the major organic product of the following reaction sequence. .CI 1) Mg, diethyl ether 2) 3)…
A: 1)We can say that the above reaction is a mode to synthesise a alcohol using a Girgnard reagent and…
Q: Predict the product and draw a stepwise mechanism for the following reaction: Part 1 HBr Br view…
A: 1-phenylethan-1-ol reacts with HBr to give (1-bromoethyl) benzene. The given chemical reaction:
Q: Draw a stepwise mechanism for the following reaction.
A: A stepwise mechanism for the following reaction is given below :
Q: Draw the MAJOR product(s) for each of the following reactions. 1) NaNH2 2) CH3I 3) 9-BBN 4) H2O2,…
A: NaNh2 is a base it abstracts hydrogen from alkyne then with ch3I nucleophillic substitution reaction…
Q: 20) Draw a stepwise, detailed mechanism for the following reaction. Use curved arrows to show the…
A:
Q: For each pair of reactants below, select the one that is more nucleophilic. 1. (a) CH3OCH3 or (b)…
A: (a) The electronegativity of Sulphur is less than the oxygen. Sulfur atom is bigger in size than the…
Q: 10.1 Draw the major product(s) each reaction below. ROOR HBr hv or A ROOR hv or A b. HBr Li. NH,
A:
Q: 15) Explain why (A) is the major product in the following reaction. HBr HBr (A) (B) Br Br
A: The Addition is based on markonikovs rule. When an Unsymmetrical reagent is added to an…
Q: 8. 1. HBr (1 equiv) 2. HCI (1 equiv) A) Br Br B) CI Br C) D) Br E) None of these choices. What is…
A: The given reaction is;
Q: 7. Draw the intermediate and final product formed in the following reaction sequence. MgBr -c-c H…
A: Please find your solution below : 7. Epoxides also known as cyclic ethers on reaction with ethanol…
Q: What products are formed in the following reaction? (CH3CH2),CuLi -CH2-CI I - CH2CH2CH3 CH3CH2CU…
A:
Q: Predict the product and draw a stepwise mechanism for the following reaction: Part 1: HBr Br HOH…
A: Applying concept of reagents and reaction.
Q: r. 1. ВНз 2. H20, H2O2, NaOH S. H2 Pd/C t. HO, H2SO4
A: Hydroboration-oxidation of alkenes that means reaction of alkenes with BH3 , H2O2-NaOH ) form less…
Q: Select the best reagents to produce the product shown as the major or exclusive product. OH O…
A: A reaction is given in the question. We have to find the reagent from the options for which the…
Q: An epoxide is formed when an aldehyde or ketone is treated with a sulfonium ylid. An example of this…
A:
Q: 68. Which reagents best accomplish this transformation? ZON Br a b C 1. Br2, FeBr3 2. H2, Pt 3.…
A: Halogenation reaction is the reaction in which addition of halogen to the reactant molecule in…
Q: Draw the most stable form of the major product in the following reaction. 1. NaOC,H, 2. Н,о* С, Н,ОН
A: Given, to draw the most stable form of the major product for the following reaction :
Q: CH-CE CH CH2-C-CH3
A:
Q: Given the follówing proposed mechar reaction. 2A+ 2B C+2D step 1: 2A+B-C+E(slow) step 2: E+B-2D…
A: In any given reaction, slowest step is the rate determining step that means rate of reaction depends…
Q: 1. 2. 3. What is the major product for the following reactions? CH₂ CH₂ HBr. H₂C ↑ CH3 CH3 CH3 -CH3…
A: In HBr Addition to an Alkene, HBr is added to alkenes to create alkyl halides. Ozonolysis is organic…
Q: Q#7 Draw a stepwise, detailed mechanism for the following reaction. Use curved arrows to show the…
A: Mechanism = To be determined
Q: 2. Give a stepwise mechanism for each reaction (i) OH 1) CH;MgBr (excess) 2) H2О (ii) OCH3 „NO2 N-…
A:
Q: Nicotine can be made when the following ammonium salt is treated with Na2CO3. Draw a stepwise…
A: The structure of ammonium salt, from which nicotine is prepared, shows that it contains a protonated…
Q: Draw the major product of each reaction. 1) Hg (Oac ) 2 4 2) Br₂ / H₂0 3) NaBHy 03 H₂/Pd m PCBA H₂0²…
A: Given: To find: the major product when the given compound(vinly cyclohexane) reacts with the…
Q: A a. Draw the structure of both products B and C of these following transformations. ( 1) O3,…
A:
Q: c) Draw the major product(s) for each reaction. 1. Sia,BH 2. Н,О2, NaOH Na, NH3 3-hexyne (c) xs I2…
A:
Q: Draw a stepwise mechanism for the following reaction
A:
Q: Select the major product of the reaction sequence below. B OCH CH 1. NaOEVEIOH 2. H3O* O OCH,CH,…
A: Applying concept claisen condensation reaction.
Q: 25. Draw a circle around the alkene that reacts most rapidly with HCl and draw a rectangle around…
A:
Q: Which reagent will accomplish the following? reagent a) PBr3 b) SOCI2 c) CrO3/ H* d) H2SO4/ heat e)…
A: We will solve this organic reaction by applying appropriate mechanism.
Q: Be sure to answer all parts. Draw a stepwise mechanism for the following reaction: HBr OH Br Part 1:…
A: In the given reaction alcohol is reacting with HBr to give alkyl halide as the product, In the given…
Q: What is the major product for the following sequence? A A X B В Your answer C D D 1. HBr 1.…
A: In step first there is overall elimination reaction and most substituted alkene formed. In step 2…
Q: Draw a stepwise mechanism for the following reaction: HBr HO, Br Part 1: HOBR Br H20 HO view…
A: Given reaction is preparation of alkyl bromide from alcohol It is an example of nucleophilic…
Q: restion 38 Which of the following reagents can accomplish the transformation below? H. HO. HO, O )…
A:
Q: Draw a stepwise, detailed mechanism for the following reaction.
A: The CH3NH2 attacks on the Cl and removes it in step 1. And then in step 2, due to presence of…
Q: Predict the product and draw a stepwise mechanism for the following reaction: Part 1: Br HBr HO view…
A: The reaction described here is a acid catalysed dehydrtion followed by nucleophilic attack on…
Q: Draw a curved arrow mechanism and predict the product. H EN CH3 H3C Create OscerSketch Answer 1…
A:
Trending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

- Draw a stepwise mechanism for the following reaction that illustrates how two substitution products are formed. Explain why 1-bromohex-2ene reacts rapidly with a weak nucleophile (CH3OH) under SN1 reaction conditions, even though it is a 1 ° alkyl halide.(a) Which compound in each of the following pairs will react faster in SN2 reaction with -OH group?(i) CH3Br or CH3I(ii) (CH3)3CCl or CH3ClWrite a stepwise mechanism for the following reactions. Please answer correct i will give you upvote but answer all correct
- This is Wittig Rxn: Whatever mechanism you choose to draw is fine since you can leave the base as B: Draw the arrow pushing mechanism using the compounds below – Constant: 4-nitrobenzyl benzaldehydebase 1: triethylaminebase 2: NaOHbase 3: K2CO3ylide: Acetonyltriphenylphosphonium chlorideDraw a stepwise mechanism for the following reaction that illustrates how two substitution products are formed. Explain why 1-bromohex-2-ene reacts rapidly with a weak nucleophile (CH3OH) under SN1 reaction conditions, even though it is a 1° alkyl halide.4-Methylpyridine reacts with benzaldehyde (C6H5CHO) in the presence of base to form A. (a) Draw a stepwise mechanism for this reaction. (b) Would you expect 2- methylpyridine or 3-methylpyridine to undergo a similar type of condensation reaction? Explain why or why not.
- Provide the suitable reagents to effect the following transformations. I specifically need help on sub-parts d, e, f, g, h, and i.4-Methylpyridine reacts with benzaldehyde (C6H5CHO) in the presence of base to form A. (a) Draw a stepwise mechanism for this reaction. (b) Would you expect 2-methylpyridine or 3-methylpyridine to undergo a similar type ofcondensation reaction? Explain why or why not.Draw a stepwise mechanism for the attached reaction that illustrateshow two substitution products are formed. Explain why 1-bromohex-2-ene reacts rapidly with a weak nucleophile (CH3OH) under SN1 reactionconditions, even though it is a 1 ° alkyl halide.
- When allyl bromide is refluxed with magnesium metal in ether solvent, the product formed is 1,5-hexadiene. (C6H10). What is the curved arrow mechanism for this reaction?Answer the following questions based on the compounds below: (pictures) a) Which compound has the lower boiling point? Explain. b) Draw the SN1 mechanism for the reaction of compound B with sodiumhydroxide, NaOH.Fill in the missing reagents below.

