Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "Submit". Use H30* for hydronium ion. Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and nickel(II) sulfide are combined. +
Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "Submit". Use H30* for hydronium ion. Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and nickel(II) sulfide are combined. +
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section: Chapter Questions
Problem 77GQ: The mineral dolomite contains magnesium carbon-ate. This reacts with hydrochloric add. MgCO3(s) + 2...
Related questions
Question
| Be sure to specify states such as (aq) or (s). Use H3O+ for hydronium ion. |
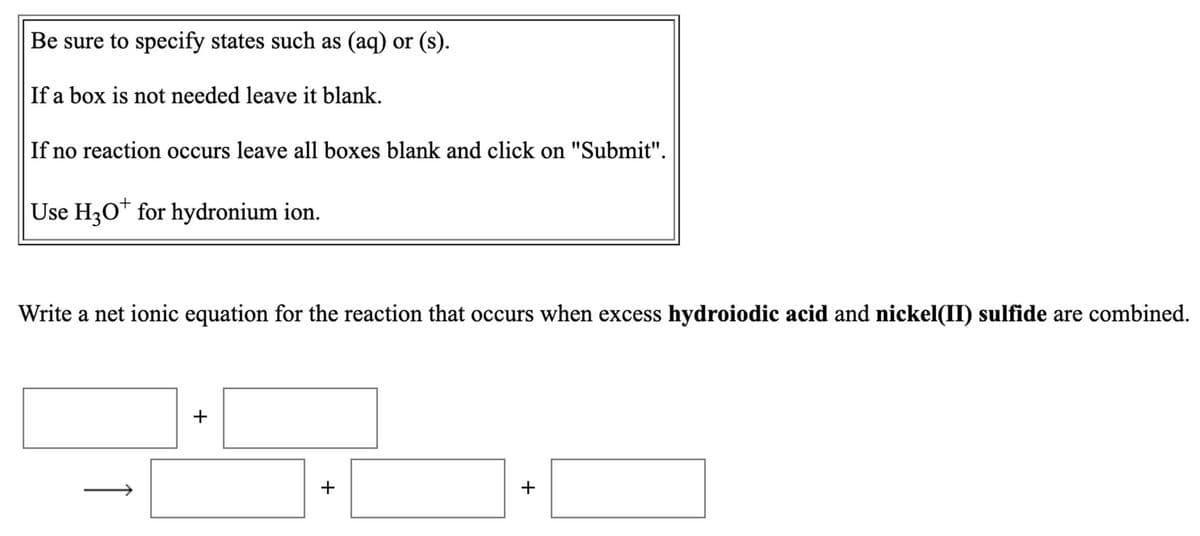
Transcribed Image Text:Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "Submit".
Use H30* for hydronium ion.
Write a net ionic equation for the reaction that occurs when excess hydroiodic acid and nickel(II) sulfide are combined.
+
+
+
1

Transcribed Image Text:Write a net ionic equation for the reaction that occurs when sodium sulfite (aq) and excess nitric acid (aq) are combined. Note: Sulfites follow the same solubility trends as sulfates.
+
+
+
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning