Be sure to specify states such as (aq) or (s). Use H3O* for the hydronium ion. If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit". Nrite a net ionic equation for the reaction that occurs when aqueous solutions of hydrobromic acid and ammonia are combined.
Be sure to specify states such as (aq) or (s). Use H3O* for the hydronium ion. If a box is not needed leave it blank. If no reaction occurs leave all boxes blank and click on "submit". Nrite a net ionic equation for the reaction that occurs when aqueous solutions of hydrobromic acid and ammonia are combined.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter9: Chemical Reactions
Section: Chapter Questions
Problem 122A: Apply Write the chemical equations and net ionic equations for each of the following reactions that...
Related questions
Question
100%
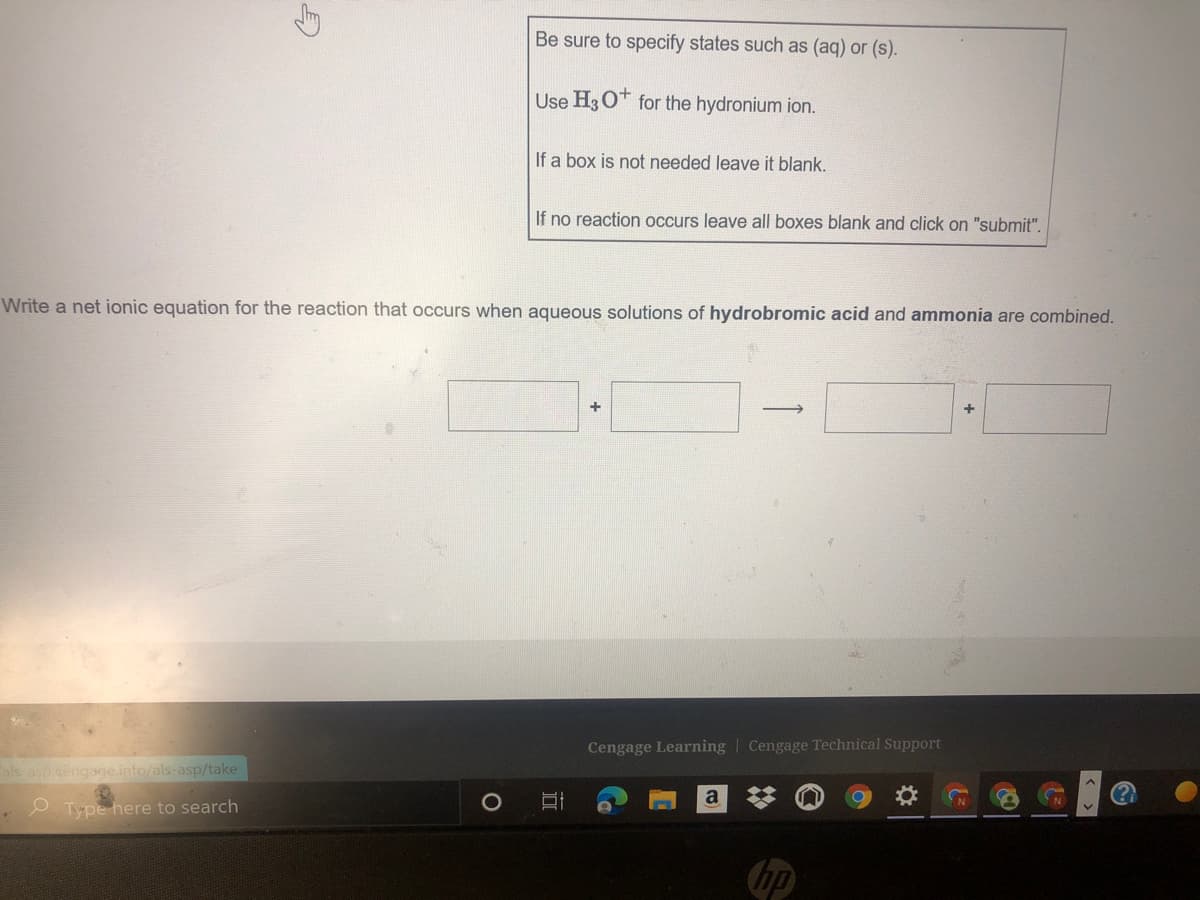
Transcribed Image Text:Be sure to specify states such as (aq) or (s).
Use H3 O* for the hydronium ion.
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "submit".
Write a net ionic equation for the reaction that occurs when aqueous solutions of hydrobromic acid and ammonia are combined.
+
+
Cengage Learning Cengage Technical Support
als asp.cengage.info/als-asp/take
e. Type here to search
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning