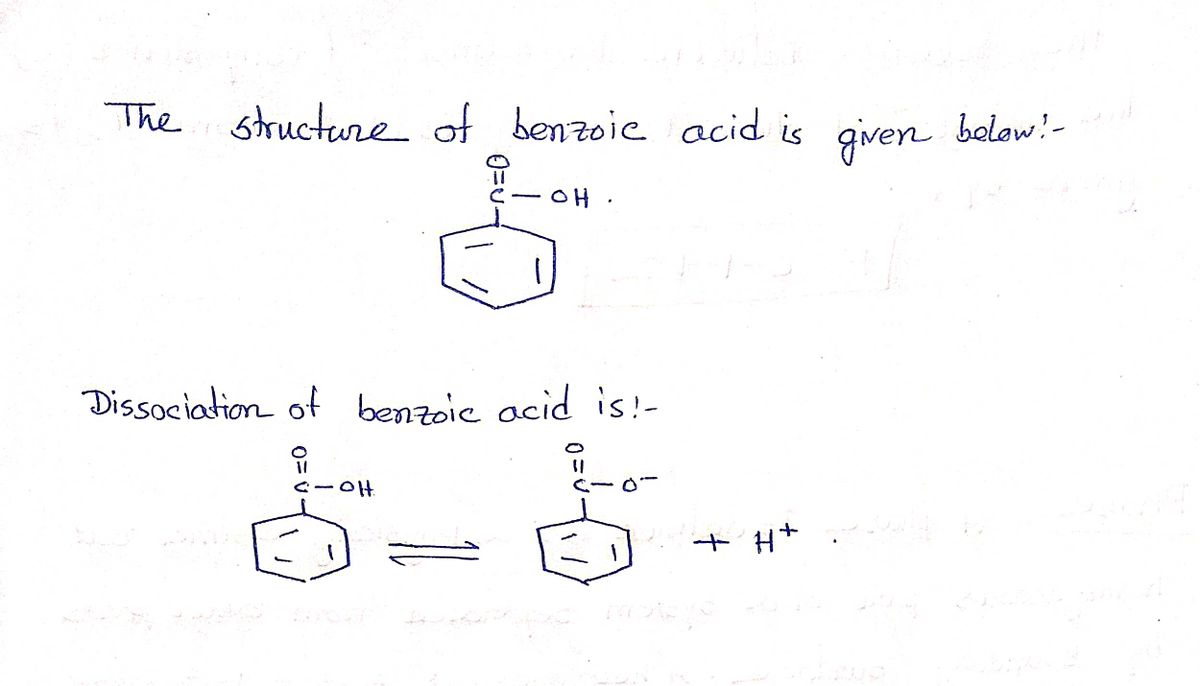
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal hygiene products. Benzoic acid dissociates in water: CH;COOH CH;C00-+H+ The pK, of this reaction is 4.2. In a 0.62 M solution of benzoic acid, what percentage of the molecules are ionized? Express the percentage numerically using two significant figures.
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications, and personal hygiene products. Benzoic acid dissociates in water: CH;COOH CH;C00-+H+ The pK, of this reaction is 4.2. In a 0.62 M solution of benzoic acid, what percentage of the molecules are ionized? Express the percentage numerically using two significant figures.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section: Chapter Questions
Problem 116IL: Amino acids are an important group of compounds. At low pH, both the carboxylic acid group (CO2H)...
Related questions
Question
Pleas help quick!! Tysm!

Transcribed Image Text:X Incorrect; Try Again
Part B
Benzoic acid is a weak acid that has antimicrobial properties. Its sodium salt, sodium benzoate, is a preservative found in foods, medications,
and personal hygiene products. Benzoic acid dissociates in water:
C,H5COOH = C,H;C00¯ +H+
The pK, of this reaction is 4.2. In a 0.62 M solution of benzoic acid, what percentage of the molecules are ionized?
Express the percentage numerically using two significant figures.
> View Available Hint(s)
VO AEd
percent ionized =
Submit
< Return to Assignment
Provide Feedback
P Pearson
2022 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions | Contact Us |
6:03 AM
2/14/2022
DELL
MLWSD000111433
F7
F8
F9
F10
F11
F12
Home
End
Pris
8.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
