Borohydride reductions of aldehydes and ketones can also be accomplished using zinc borohydride, Zn(BH 4 )2. Consider the ketoester shown below. In theory, what is the minimum mass of Zn(BH 4 ) 2 required to fully reduce 9.32 g of the ketoester above?
Borohydride reductions of aldehydes and ketones can also be accomplished using zinc borohydride, Zn(BH 4 )2. Consider the ketoester shown below. In theory, what is the minimum mass of Zn(BH 4 ) 2 required to fully reduce 9.32 g of the ketoester above?
Chapter3: Mechanisms
Section: Chapter Questions
Problem 152EQ
Related questions
Question
Borohydride reductions of
using zinc borohydride, Zn(BH 4 )2. Consider the ketoester shown below.
In theory, what is the minimum mass of Zn(BH 4 ) 2 required to fully reduce
9.32 g of the ketoester above?
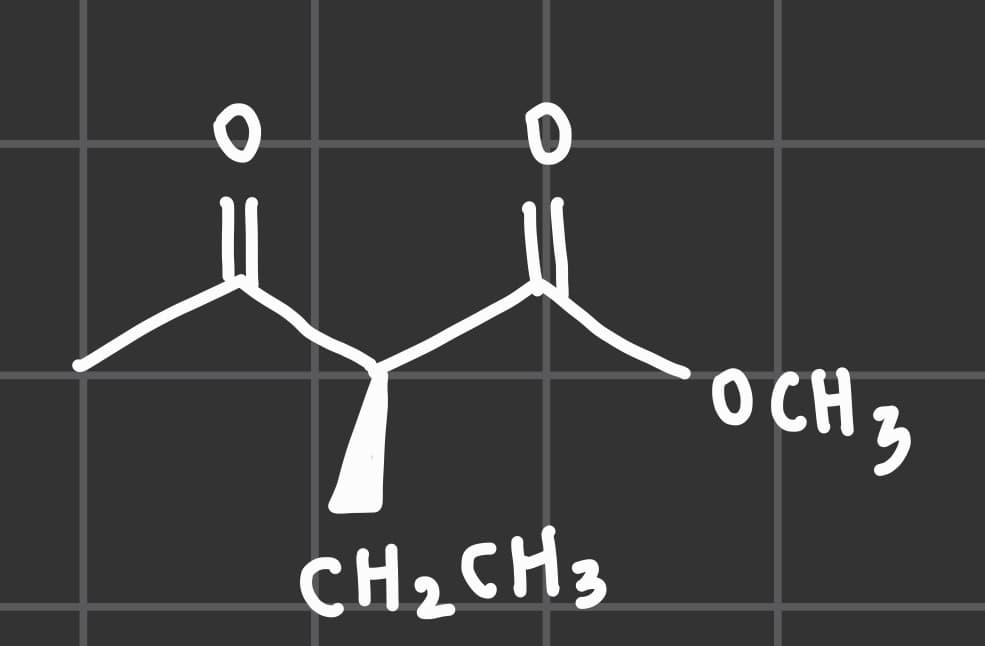
Transcribed Image Text:0
0
CH₂ CH3
OCH 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
how did you know that for every 1 mol of ketoester, we need 2 moles of zinc borohydride?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

