Chapter3: Mechanisms
Section: Chapter Questions
Problem 96EQ
Related questions
Question
a).
) Write the chemical formula for each compound.
b) Order the compounds from lowest to highest boiling point and explain your reasoning in great detail. Use the concept of London dispersion forces to rationalize your answer clearly explaining what such forces are.
c) Of the atoms contained in the molecules above, order them in sequence from least to most polarizable and clearly explain your reasoning.
d) Which molecule do you think is the most viscous and why?
e) In the presence of acetic acid, which molecules above have the potential to engage in hydrogen bonding and why?
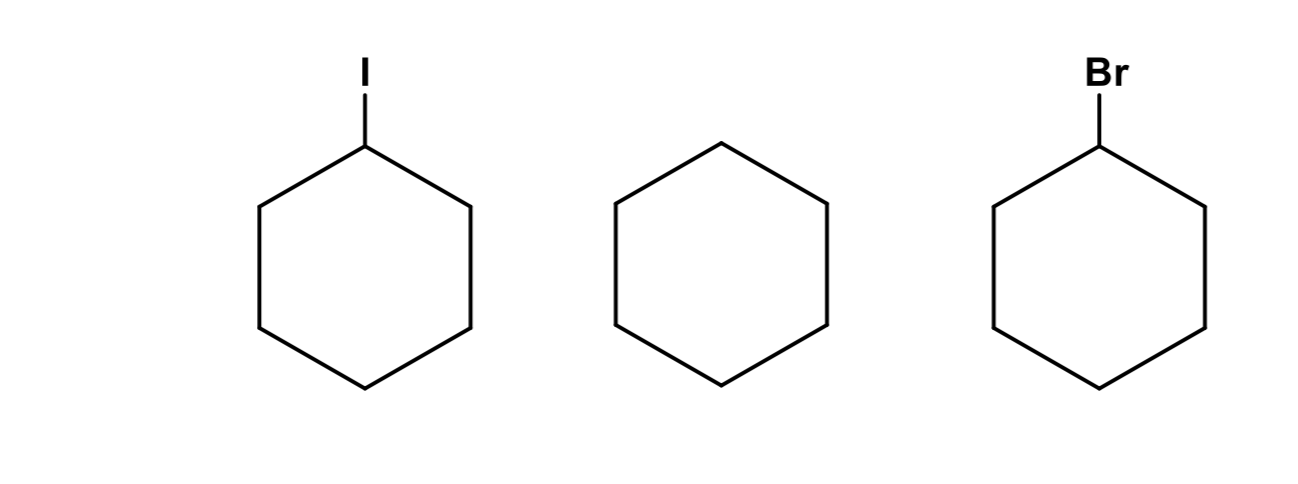
Transcribed Image Text:Br
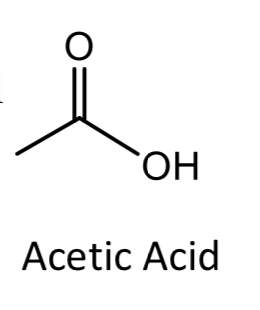
Transcribed Image Text:"ОН
Acetic Acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

