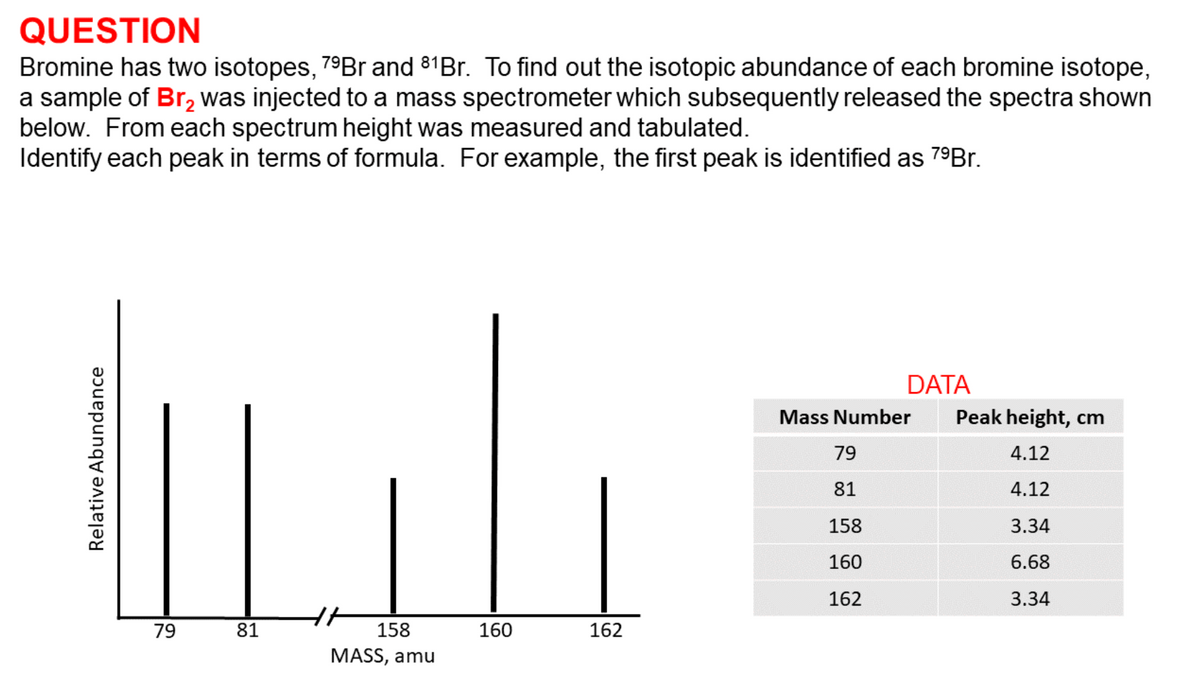
Bromine has two isotopes, 79Br and 81Br. To find out the isotopic abundance of each bromine isotope, a sample of Br, was injected to a mass spectrometer which subsequently released the spectra shown below. From each spectrum height was measured and tabulated. Identify each peak in terms of formula. For example, the first peak is identified as 79Br. DATA Mass Number Peak height, cm 79 4.12 81 4.12 158 3.34 160 6.68 162 3.34 79 81 158 160 162 MASS, amu Relative Abundance
Bromine has two isotopes, 79Br and 81Br. To find out the isotopic abundance of each bromine isotope, a sample of Br, was injected to a mass spectrometer which subsequently released the spectra shown below. From each spectrum height was measured and tabulated. Identify each peak in terms of formula. For example, the first peak is identified as 79Br. DATA Mass Number Peak height, cm 79 4.12 81 4.12 158 3.34 160 6.68 162 3.34 79 81 158 160 162 MASS, amu Relative Abundance
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL2: Mass Spectrometry
Section: Chapter Questions
Problem 27CTQ
Related questions
Question
Transcribed Image Text:QUESTION
Bromine has two isotopes, 79Br and 81Br. To find out the isotopic abundance of each bromine isotope,
a sample of Br, was injected to a mass spectrometer which subsequently released the spectra shown
below. From each spectrum height was measured and tabulated.
Identify each peak in terms of formula. For example, the first peak is identified as 79Br.
DATA
Mass Number
Peak height, cm
79
4.12
81
4.12
158
3.34
160
6.68
162
3.34
79
81
158
160
162
MASS, amu
Relative Abundance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning