Buffers are aqueous solutions that tend to resist changes in pH when small amounts of strong acid (H+) or base (OH-) are added. Compare the changes in the pH of the resulting solution when you add 0.1 M NaOH in (a) distilled water and (b) bicarbonate solution. O A) No changes in pH of both solutions. O B) (a) and (b) solutions will have the same pH. O c) pH change in (b) will be higher than in (a). D) pH change in (a) will be higher than in (b).
Buffers are aqueous solutions that tend to resist changes in pH when small amounts of strong acid (H+) or base (OH-) are added. Compare the changes in the pH of the resulting solution when you add 0.1 M NaOH in (a) distilled water and (b) bicarbonate solution. O A) No changes in pH of both solutions. O B) (a) and (b) solutions will have the same pH. O c) pH change in (b) will be higher than in (a). D) pH change in (a) will be higher than in (b).
Chapter16: Biochemistry And Biotechnology
Section: Chapter Questions
Problem 8E
Related questions
Question
15 29

Transcribed Image Text:Buffers are aqueous solutions that tend to resist changes in pH when small amounts of
strong acid (H+) or base (OH-) are added. Compare the changes in the pH of the resulting
solution when you add 0.1 M NAOH in
(a) distilled water and
(b) bicarbonate solution.
O A) No changes in pH of both solutions.
O B) (a) and (b) solutions will have the same pH.
O C) pH change in (b) will be higher than in (a).
O D) pH change in (a) will be higher than in (b).
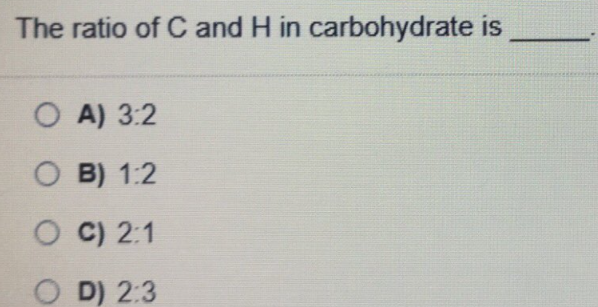
Transcribed Image Text:The ratio of C and H in carbohydrate is
O A) 3:2
O B) 1:2
O C) 2:1
O D) 2:3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry In Focus
Chemistry
ISBN:
9781305084476
Author:
Tro, Nivaldo J., Neu, Don.
Publisher:
Cengage Learning