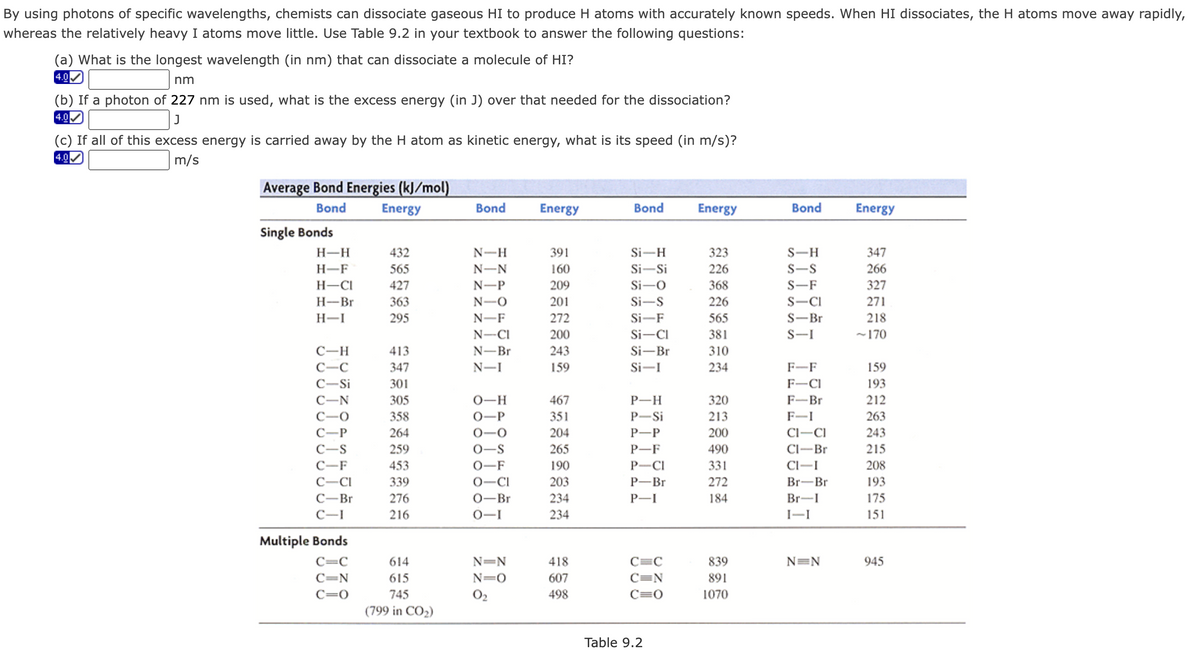
By using photons of specific wavelengths, chemists can dissociate gaseous HI to produce H atoms with accurately known speeds. When HI dissociates, the H atoms move away rapidly, whereas the relatively heavy I atoms move little. Use Table 9.2 in your textbook to answer the following questions: (a) What is the longest wavelength (in nm) that can dissociate a molecule of HI? nm (b) If a photon of 227 nm is used, what is the excess energy (in J) over that needed for the dissociation? 4.0 (c) If all of this excess energy is carried away by the H atom as kinetic energy, what is its speed (in m/s)? 4.0 m/s Aueago Rend Enoxgios (ll (mel)
By using photons of specific wavelengths, chemists can dissociate gaseous HI to produce H atoms with accurately known speeds. When HI dissociates, the H atoms move away rapidly, whereas the relatively heavy I atoms move little. Use Table 9.2 in your textbook to answer the following questions: (a) What is the longest wavelength (in nm) that can dissociate a molecule of HI? nm (b) If a photon of 227 nm is used, what is the excess energy (in J) over that needed for the dissociation? 4.0 (c) If all of this excess energy is carried away by the H atom as kinetic energy, what is its speed (in m/s)? 4.0 m/s Aueago Rend Enoxgios (ll (mel)
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.102PAE
Related questions
Question
Transcribed Image Text:By using photons of specific wavelengths, chemists can dissociate gaseous HI to produce H atoms with accurately known speeds. When HI dissociates, the H atoms move away rapidly,
whereas the relatively heavy I atoms move little. Use Table 9.2 in your textbook to answer the following questions:
(a) What is the longest wavelength (in nm) that can dissociate a molecule of HI?
4.0
nm
(b) If a photon of 227 nm is used, what is the excess energy (in J) over that needed for the dissociation?
4.0
(c) If all of this excess energy is carried away by the H atom as kinetic energy, what is its speed (in m/s)?
4.0
m/s
Average Bond Energies (kJ/mol)
Bond
Energy
Bond
Energy
Bond
Energy
Bond
Energy
Single Bonds
H-H
432
N-H
391
Si-H
323
S-H
347
H-F
565
N-N
160
Si-Si
226
S-S
266
H-CI
427
N-P
209
Si-O
368
S-F
327
Н-Br
363
N-O
201
Si-S
226
S-CI
271
H-I
295
N-F
272
Si-F
565
S-Br
218
N-CI
200
Si-CI
381
S-I
~170
310
234
C-H
413
N-Br
243
Si-Br
C-C
347
N-I
159
Si-
F-F
159
C-Si
301
F-CI
193
305
0-H
467
P-H
320
F-Br
212
C-O
358
0-P
351
P-Si
213
F-I
263
С—Р
264
0-0
204
Р—Р
200
Cl-CI
243
C-S
259
0-S
265
P-F
490
Cl-Br
215
C-F
453
0-F
190
P-CI
331
Cl-I
208
C-CI
339
0-CI
203
P-Br
272
Br-Br
193
С—Br
276
0-Br
234
P-I
184
Br-I
175
216
0-I
234
I-I
151
Multiple Bonds
C=C
614
N=N
418
C=C
839
N=N
945
C=N
615
N=0
607
C=N
891
C=0
745
O2
498
C=0
1070
(799 in CO2)
Table 9.2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
