(c) Calculate the amount of energy released per gram from each type of food tested. A (d) How did the different types of food compare? Was your prediction correct? Explain your answer. A
(c) Calculate the amount of energy released per gram from each type of food tested. A (d) How did the different types of food compare? Was your prediction correct? Explain your answer. A
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter3: Matter-properties And Changes
Section: Chapter Questions
Problem 11STP
Related questions
Question
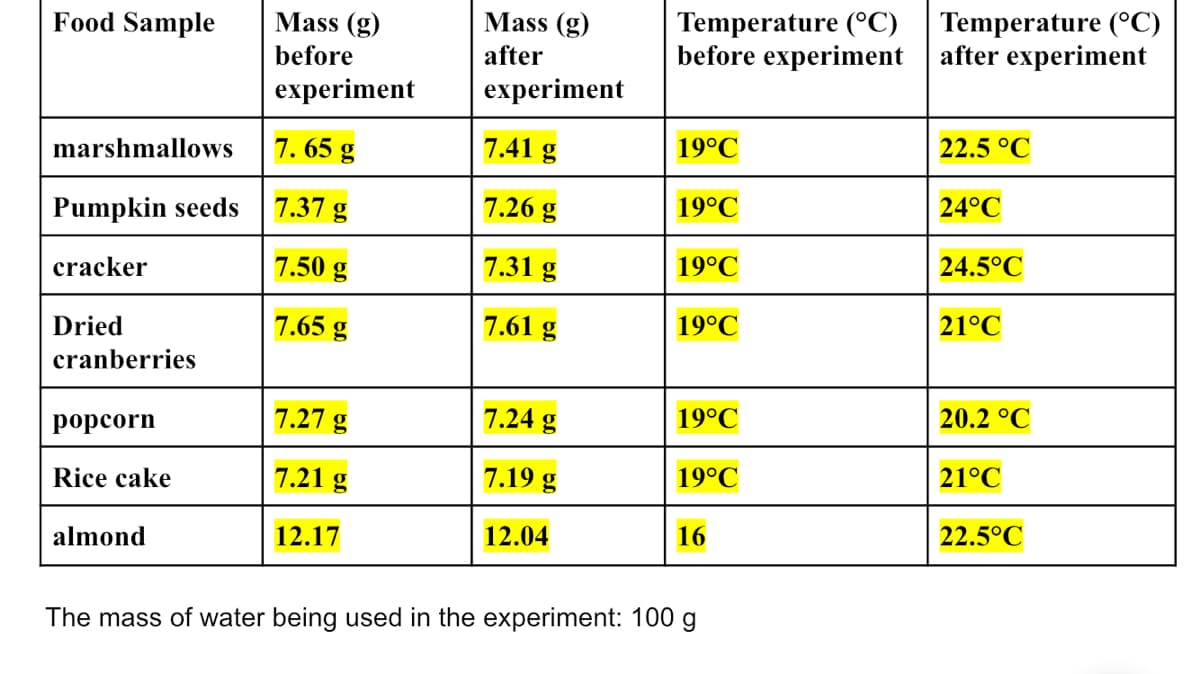
Transcribed Image Text:Temperature (°C)
before experiment
Temperature (°C)
after experiment
Food Sample
Mass (g)
Mass (g)
before
after
experiment
experiment
marshmallows
7. 65 g
7.41 g
19°C
22.5 °C
7.37 g
7.26 g
19°C
24°C
Pumpkin seeds
7.50 g
7.31 g
19°C
24.5°C
cracker
Dried
7.65 g
7.61 g
19°C
21°C
cranberries
7.27 g
7.24 g
19°C
20.2 °C
рорсorn
7.21 g
7.19 g
19°C
21°C
Rice cake
12.17
12.04
16
22.5°C
almond
The mass of water being used in the experiment: 100 g

Transcribed Image Text:(c) Calculate the amount of energy released per gram
from each type of food tested.
(d) How did the different types of food compare? Was
your prediction correct? Explain your answer. A
(e) Foods cannot be compared without determining the
"per gram" values. Explain the reasoning behind this
statement. A
(f) Explain your results in terms of the types of bonds
and the relative bond energies found in carbohydrates,
lipids, and proteins.
(g) Describe what needed to be done to each food sample
before it began to react on its own. Why is this not an
option in living cells?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax